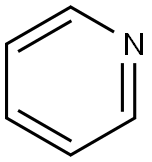
Pyridine
- CAS No.
- 110-86-1
- Chemical Name:
- Pyridine
- Synonyms
- PY;AA;PYR;Azine;2-PROPENOL;azabenzene;Pure pyridine;Pyridine anhydrous;Piridina;FEMA 2966
- CBNumber:
- CB8852825
- Molecular Formula:
- C5H5N
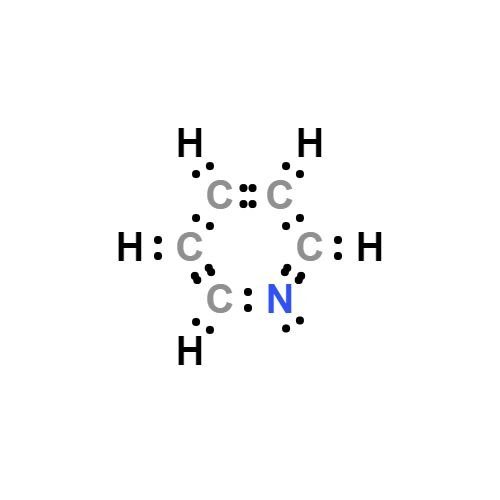
Lewis structure
- Molecular Weight:
- 79.1
- MDL Number:
- MFCD00011732
- MOL File:
- 110-86-1.mol
- MSDS File:
- SDS
| Melting point | -42 °C (lit.) |
|---|---|
| Boiling point | 115 °C (lit.) |
| Density | 0.978 g/mL at 25 °C (lit.) |
| vapor density | 2.72 (vs air) |
| vapor pressure | 23.8 mm Hg ( 25 °C) |
| FEMA | 2966 | PYRIDINE |
| refractive index |
n |
| Flash point | 68 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: in accordance |
| form | Liquid |
| pka | 5.25(at 25℃) |
| color | colorless |
| Odor | Nauseating odor detectable at 0.23 to 1.9 ppm (mean = 0.66 ppm) |
| Relative polarity | 0.302 |
| PH | 8.81 (H2O, 20℃) |
| explosive limit | 12.4% |
| Odor Threshold | 0.063ppm |
| Odor Type | fishy |
| Water Solubility | Miscible |
| FreezingPoint | -42℃ |
| λmax |
λ: 305 nm Amax: 1.00 λ: 315 nm Amax: 0.15 λ: 335 nm Amax: 0.02 λ: 350-400 nm Amax: 0.01 |
| Merck | 14,7970 |
| BRN | 103233 |
| Henry's Law Constant | 18.4 at 30 °C (headspace-GC, Chaintreau et al., 1995) |
| Dielectric constant | 12.5(20℃) |
| Exposure limits | TLV-TWA 5 ppm (~15 mg/m3) (ACGIH, MSHA,and OSHA); STEL 10 ppm (ACGIH), IDLH 3600 ppm (NIOSH). |
| Stability | Stable. Flammable. Incompatible with strong oxidizing agents, strong acids. |
| InChIKey | JUJWROOIHBZHMG-UHFFFAOYSA-N |
| LogP | 0.64 at 20℃ |
| Substances Added to Food (formerly EAFUS) | PYRIDINE |
| FDA 21 CFR | 177.1580 |
| CAS DataBase Reference | 110-86-1(CAS DataBase Reference) |
| EWG's Food Scores | 9 |
| FDA UNII | NH9L3PP67S |
| Proposition 65 List | Pyridine |
| IARC | 2B (Vol. 77, 119) 2019 |
| NIST Chemistry Reference | Pyridine(110-86-1) |
| EPA Substance Registry System | Pyridine (110-86-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302+H312+H332-H315-H319 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | T,N,F,Xn | |||||||||
| Risk Statements | 11-20/21/22-39/23/24/25-23/24/25-52-36/38 | |||||||||
| Safety Statements | 36/37/39-38-45-61-28A-26-28-24/25-22-36/37-16-7 | |||||||||
| RIDADR | UN 1282 3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | UR8400000 | |||||||||
| F | 3-10 | |||||||||
| Autoignition Temperature | 482 °C | |||||||||
| Hazard Note | Highly Flammable/Harmful | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2933 31 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in rats: 1.58 g/kg (Smyth) | |||||||||
| IDLA | 1,000 ppm | |||||||||
| NFPA 704 |
|
Pyridine price More Price(113)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W296600 | Pyridine ≥99% | 110-86-1 | 1kg | $89.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | W296600 | Pyridine ≥99% | 110-86-1 | 10Kg | $566 | 2024-03-01 | Buy |
| Sigma-Aldrich | W296600 | Pyridine ≥99% | 110-86-1 | 25kg | $944 | 2024-03-01 | Buy |
| Sigma-Aldrich | PX2020 | Pyridine Meets ACS Specifications, Meets Reagent Specifications for testing USP/NF monographs GR ACS | 110-86-1 | 100mL | $132 | 2024-03-01 | Buy |
| Sigma-Aldrich | PX2020 | Pyridine Meets ACS Specifications, Meets Reagent Specifications for testing USP/NF monographs GR ACS | 110-86-1 | 500mL | $193 | 2024-03-01 | Buy |
Pyridine Chemical Properties,Uses,Production
Chemical Structure
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. The pyridine ring occurs in many important compounds, including azines and the vitamins niacin and pyridoxine.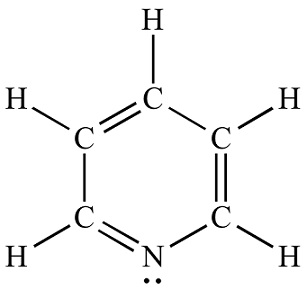
Pyridine Lewis structure
Chemical properties
Pyridine is a colourless flammable liquid with a strong and unpleasant fish-like odour.
Pyridine
Productions
2.1 Separation from Tar
Pyridine bases are a constituent of tars. They were isolated from coal tar or coal gas before synthetic manufacturing processes became established. The amounts contained in coal tar and coal gas is small, and the pyridine bases isolated from them are a mixture of many components. Thus, with a few exceptions, isolation of pure pyridine bases was expensive. Today, almost all pyridine bases are produced by synthesis.2.2 Chichibabin synthesis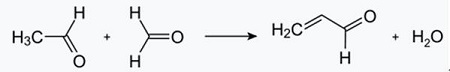
Fig. 2-1 Formation of acrolein from acetaldehyde and formaldehyde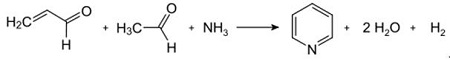
Fig. 2-2 Condensation of pyridine from acrolein and acetaldehyde
The Chichibabin pyridine synthesis was reported in 1924 and is still in use in industry. Acetaldehyde and formaldehyde react with ammonia to give mainly pyridine. First, acrolein is formed in a Knoevenagel condensation from the acetaldehyde and formaldehyde. It is then condensed with acetaldehyde and ammonia into dihydropyridine, and then oxidized with a solid-state catalyst to pyridine. The reaction is usually carried out at 350-550°C and a space velocity of 500-1000 h -1 in the presence of a solid acid catalyst (e.g., silica-alumina). The product consists of a mixture of pyridine, simple methylated pyridines (picoline), and lutidine. The recovered pyridine is separated from byproducts in a multistage process.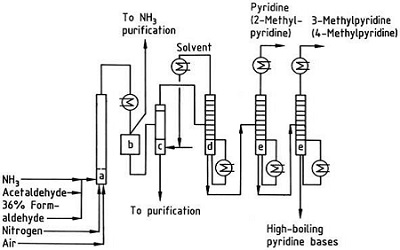
Fig. 2-3 Flow sheet of pyridine and methylpyridine production from acetaldehyde and formaldehyde with ammonia. A) Reactor; b) Collector; c) Extraction; d) Solvent distillation; e) Distillation
2.3 Dealkylation of Alkylpyridines
Pyridine can be prepared by dealkylation of alkylated pyridines, which are obtained as byproducts in the syntheses of other pyridines. The oxidative dealkylation is carried out either using air over vanadium(V) oxide catalyst, by vapor-dealkylation on nickel-based catalyst, or hydrodealkylation with a silver- or platinum-based catalyst. Yields of pyridine up to be 93% can be achieved with the nickel-based catalyst.
2.4 Synthesis from Nitriles and Acetylene
Liquid-phase reaction of nitriles with acetylene is carried out at 120-180 ?C and 0.8-2.5 MPa in the presence of an organocobalt catalyst and gives 2-substituted pyridines: 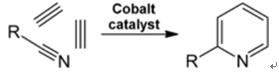
Fig. 2-4 Synthesis of 2-methylpyridine from nitriles and acetylene
The trimerization of a part of a nitrile molecule and two parts of acetylene into pyridine is called Bönnemann cyclization. When using acetonitrile as the nitrile, 2-methylpyridine is obtained, which can be dealkylated to pyridine.
2.5 Synthesis from Acrylonitrile and Ketones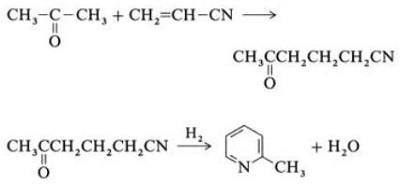
Fig. 2-5 Synthesis of 2-methylpyridine from acrylonitrile and acetone
Synthesis from acrylonitrile and acetone gives 2-methylpyridine selectively, which can be dealkylated to pyridine. First, the reaction of acrylonitrile and acetone, catalyzed by a primary aliphatic amine such as isopropylamine and a weak acid such as benzoic acid, occurs in the liquid phase at 180 ?C and 2.2 MPa to give 5-oxohexanenitrile, with 91% selectivity. The acrylonitrile conversion is 86%. Then cyclization and dehydration of the initial product are carried out in the gas phase in the presence of hydrogen over a palladium, nickel, or cobalt-containing catalyst at 240?C to give 2-methylpyridine in 84% yield.
2.6 Synthesis from Dinitriles
In a vapor-phase reaction over a nickel-containing catalyst in the presence of hydrogen, 2-methylglutaronitrile gives 3-methylpiperidine, which then undergoes dehydrogenation over palladium –alumina to give 3-methylpyridine. And 3-methylpyridine also can be dealkylated to pyridine.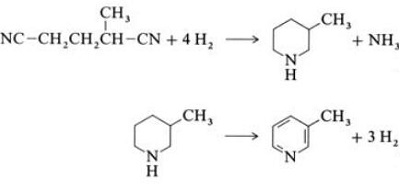
Fig. 2-6 Synthesis of 2-methylpyridine from Dinitriles
A one-step gas-phase reaction over a palladium-containing catalyst is reported to give 3-methylpyridine in 50% yield.
2.7 Biosynthesis
Several pyridine derivatives play important roles in biological systems. While its biosynthesis is not fully understood, nicotinic acid (vitamin B3) occurs in some bacteria, fungi, and mammals. Mammals synthesize nicotinic acid through oxidation of the amino acid tryptophan, where an intermediate product, aniline, creates a pyridine derivative, kynurenine. On the contrary, the bacteria Mycobacterium tuberculosis and Escherichia coli produce nicotinic acid by condensation of glyceraldehyde 3-phosphate and aspartic acid.
2.8 Other methods
Ethylene and ammonia react in the presence of a palladium complex catalyst to give 2-methylpyridine and MEP. Pyridine can be prepared from cyclopentadiene by ammoxidation, or from 2-pentenenitrile by cyclization and dehydrogenation. Furfuryl alcohol or furfural reacts with ammonia in the gas phase to give pyridine. 2-Methylpyridine is also prepared from aniline.
Uses
3.1 Solvent
Pyridine(110-86-1) is a polar, basic, low-reactive solvent, especially for dehydrochlorination reactions and extraction of antibiotics. In elimination reaction, pyridine acts as the base of the elimination reaction and bonds the resulting hydrogen halide to form a pyridinium salt. In esterifications and acylations, pyridine activates the carboxylic acid halides or anhydrides.
3.2 Medicines
Pyridine's chemical structure can be found in various medications that are synthesized thanks in part to pyridine. One example is a medication called esomeprazole, the generic name for Nexium. This is a medication that's used to treat GERD, or gastroesophageal reflux disease. Another example of a pyridine containing medication is loratadine, more commonly known by its brand name of Claritin. Loratadine helps in the treatment of allergies.
3.3 Pesticides
The main use of pyridine is as a precursor to the herbicides paraquat and diquat. The first synthesis step of insecticide chlorpyrifos consists of the chlorination of pyridine. Pyridine is also the starting compound for the preparation of pyrithione-based fungicides. Cetylpyridinium and laurylpyridinium, which can be produced from pyridine with a Zincke reaction, are used as antiseptic in oral and dental care products. Pyridine is easily attacked by alkylating agents to give N-alkylpyridinium salts. One example is cetylpyridinium chloride.
Fig 3-1 Synthesis of paraquat
3.4 Synthesis of piperidine
Piperidine, a fundamental nitrogen heterocycle, is important synthetic building-block. Piperidines are produced by hydrogenation of pyridine with a nickel-, cobalt-, or ruthenium-based catalyst at elevated temperatures.
C5H5N + 3 H2 → C5H10NH3.5 Ligand and Lewis base
Pyridine is widely used as a ligand in coordination chemistry. As a ligand of metal complex, it can be easily replaced by a stronger Lewis base, which can be used in the catalysis of polymerization and hydrogenation reactions. After the completion of reaction, pyridine ligand replaced during the reaction can be restored again. Pyridine is also used as a base in condensation reactions. As a base, pyridine can be used as the Karl Fischer reagent, but it is usually replaced by alternatives with a more pleasant odor, such as imidazole.
3.6 Others
Except for above uses, Pyridine is also used to product polycarbonate resins, vitamins, food flavorings, paints, dyes, rubber products, adhesives, and waterproofing for fabrics. Pyridine is added to ethanol to make it unsuitable for drinking. It is also used in the in vitro synthesis of DNA.
Toxicity information
4.1 Toxicity Level
Low Toxicity
4.2 Acute Toxicity
LD501580mg/kg (Large mice, oral); 1121mg/kg (Rabbit, through skin); inhaled by human 25mg/m3×20 min, irritation of conjunctiva and upper respiratory tract mucosa. Subacute and chronic toxicity: inhaled by large mice 32.3mg/m3×7 hours/day x5 days/week x6 months, increase in liver weight; inhaled by humans 20~40mg/m3 (long term), nerve damage, unsteady walking, digital tremors, low blood pressure, over-sweating, occasional liver and kidney damage.
Hazards
5.1 Health hazards
Pyridine is extremely toxic by ingestion and inhalation. Vapors are heavier than air. its combustion produces toxic oxides of nitrogen. Pyridine is highly flammable (its flash point is just 17 ºC). Pyridine also could have neurotoxic and genotoxic effects.
5.2 Fire Hazards
Behavior in Fire: Vapor is heavier than air and may travel considerable distance to source of ignition and flash back.
References
- https://en.wikipedia.org/wiki/Pyridine#Occurrence
- http://www.zwbk.org/MyLemmaShow.aspx?zh=zh-tw&lid=169038
- http://www.softschools.com/formulas/chemistry/pyridine_formula/378/
- http://www.hmdb.ca/metabolites/HMDB0000926
- https://study.com/academy/lesson/pyridine-in-medicine-uses-synthesis.html#partialRegFormModal
- http://www.toxipedia.org/display/toxipedia/Pyridine
- https://www.chemicalbook.com/ProductChemicalPropertiesCB8852825_EN.htm
- https://pubchem.ncbi.nlm.nih.gov/compound/pyridine#section=Top
- http://www.ebi.ac.uk/chebi/searchId.do;jsessionid=E7088896622D62FC650863C2AD197CAA?chebiId=CHEBI:16227
- https://www.britannica.com/science/pyridine
- Shimizu, S.; Watanabe, N.; Kataoka, T.; Shoji, T.; Abe, N.; Morishita, S.; Ichimura, H. (2005), "Pyridine and Pyridine Derivatives", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a22_399
Chemical Properties
Pyridine, is a slightly yellow or colorless liquid; hygroscopic; unpleasant odor; burning taste; slightly alkaline in reaction; soluble in water, alcohol, ether, benzene, and fatty oils; specific gravity, 0.978; autoignition temperature, 482 °C. Pyridine, a tertiary amine, is a somewhat stronger base than aniline and readily forms quaternary ammonium salts.
Chemical Properties
Pyridine is a weak base (pKa= 5.25); a 0.2 M solution has a pH of 8.5 (HSDB 1988). Its carbon atoms are deactivated towards electrophilic substitution. This is especially true in acidic media, where salts form at the nitrogen. It does, however, readily undergo nucleophilic substitution, preferentially at the C-2 and also at the C-4 position (Jori et al 1983). Being a tertiary amine, pyridine reacts with alkylating agents to form quaternary salts (Santodonato et al 1985). Because of its reduced capacity to donate electrons, it is more resistant to oxidation than benzene. Oxidation with peroxy acids forms pyridine N-oxide which is then capable of undergoing electrophilic substitution (Jori et al 1983). Pyridine reacts violently with a number of compounds, including nitric acid, sulfuric acid, maleic anhydride, perchromate, beta-propiolactone and chlorosulfonic acid. Thermal decomposition can liberate cyanides (Gehring 1983). Both the pyridinium ion and pyridine itself are readily reduced to the commercially important compound, piperidine (Jori et al 1983).
Physical properties
Clear, colorless to pale yellow, flammable liquid with a sharp, penetrating, nauseating fish-like odor. Odor threshold concentrations in water and air were 2 ppm (Buttery et al., 1988) and 21 ppbv (Leonardos et al., 1969), respectively. Detection odor threshold concentrations of 0.74 mg/m3 (2.3 ppmv) and 6 mg/m3 (1.9 ppmv) were experimentally determined by Katz and Talbert (1930) and Dravnieks (1974), respectively. Cometto-Mu?iz and Cain (1990) reported an average nasal pungency threshold concentration of 1,275 ppmv.
Occurrence
Pyridine was discovered by Anderson in coal tar in 1846 (Windholz et al 1983). It is found in tobacco smoke (Vohl and Eulenberg 1871; Lehmann 1909) and roasted coffee (Bertrand and Weisweiller 1913). Pyridine is found in wood oil and in the leaves and roots of Atropa belladonna (HSDB 1988), and is also a component of creosote oil (Krone et al 1986). In nature, pyridine and its derivatives are commonly found as components of alkaloids, vitamins, and coenzymes.
Uses
Pyridine is used as a solvent in paint andrubber industries; as an intermediate in dyesand pharmaceuticals; for denaturing alcohol;and as a reagent for cyanide analysis. Itoccurs in coal tar.
Uses
Pyridine is used directly in the denaturation of alcohol (ACGIH 1986; HSDB 1989; NSC 1978) and as a solvent in paint and rubber preparation (ACGIH 1986; HSDB 1989; NSC 1978) and in research laboratories for functions such as extracting plant hormones (Santodonato et al. 1985). Half of the pyridine produced today is used as an intermediate in making various insecticides and herbicides for agricultural applications (ACGIH 1986; Harper et al. 1985; Santodonato et al. 1985). Approximately 20% goes into the production of piperidine (Harper et al. 1985; Santodonato et al. 1985) which is commercially significant in the preparation of chemicals used in rubber vulcanization and agriculture (NSC 1978). Pyridine is also used as an intermediate in the preparation of drugs (antihistamines, steroids, sulfa-type and other antibacterial agents) dyes, water repellents, and polycarbonate resins (ACGIH 1986; Harper et al. 1985; NSC 1978; Santodonato et al. 1985). Pyridine is also approved by the Food and Drug Administration (FDA) for use as a flavoring agent in the preparation of foods (Harper et al. 1985; HSDB 1989) .
Preparation
Pyridine is produced either by isolation from natural sources such as coal, or through chemical synthesis (HSDB 1989). Pyridine is produced by the fractional distillation of coal-tar residues (HSDB 1989; NSC 1978; Santodonato et al. 1985) in which 1 ton of coal produces 0.07-0.21 pounds of pyridine bases of which 57% is pyridine (Santodonato et al, 1985). Synthetically produced pyridine is currently the more important source of pyridine for commercial uses (Santodonato et al. 1985). Small amounts of pyridine are synthesized from acetaldehyde, formaldehyde, and ammonia with a fluidized silica-alumina catalyst, followed by fractionation to isolate the pyridine (Harper et al. 1985; HSDB 1989; NSC 1978).
Pyridine is produced from natural sources by Crowley Tar Products of Stow, Ohio, and Oklahoma City, Oklahoma (Harper et al. 1985; HSDB 1989; SRI 1986, 1987, 1988). Pyridine is synthetically produced by two companies, the Nepera Chemical Co. of Harriman, New York and the Reilly Tar and Chemical Corporation of Indianapolis, Indiana (Harper et al. 1985; SRI 1986, 1987, 1988).
Production Methods
Pyridine is produced from the gases obtained by the coking of coal and by direct synthesis. The light-oil fraction of coal tar is treated with sulfuric acid to produce water-soluble pyridine salts and then the pyridine bases are recovered from the aqueous phase by sodium hydroxide or ammonia (Jori et al 1983). The majority of U.S. production is through synthetic means. This process uses a vapor-phase reaction of acetaldehyde, formaldehyde and ammonia, which yields a mixture of pyridine and 3-methylpyridine (Santodonato et al 1985). The product ratio depends on the relative amounts of acetaldehyde and formaldehyde. Added methanol increases the yield. The U.S. production of pyridine was estimated at 32 to 47 million pounds in 1975 (Reinhardt and Brittelli 1981). Pyridine is commercially available in technical, 2° and 1° grades, the latter two referring to their boiling ranges. Major impurities are higher boiling homologues, such as picolines, lutidines and collidines, which are mono-, di-, and trimethylpyridines (Santodonato et al 1985; Jori et al 1983).
Definition
ChEBI: Pyridine is an azaarene comprising a benzene core in which one -CH group is replaced by a nitrogen atom. It is the parent compound of the class pyridines.The molecules have a hexagonal planar ring and are isoelectronic with benzene. Pyridine is an example of an aromatic heterocyclic compound, with the electrons in the carbon–carbon pi bonds and the lone pair of the nitrogen delocalized over the ring of atoms. The compound is extracted from coal tar and used as a solvent and as a raw material for organic synthesis.
Aroma threshold values
Detection: 0.079 to 790 ppb; recognition: 7.9 to 40 ppm
General Description
A clear colorless to light yellow liquid with a penetrating nauseating odor. Density 0.978 g / cm3. Flash point 68°F. Vapors are heavier than air. Toxic by ingestion and inhalation. Combustion produces toxic oxides of nitrogen.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
Azabenzene is a base. Reacts exothermically with acids. During preparation of a complex of Azabenzene with chromium trioxide, an acid, the proportion of chromium trioxide was increased. Heating from this acid-base reaction led to an explosion and fire [MCA Case History 1284 1967]. A 0.1% solution of Azabenzene (or other tertiary amine) in maleic anhydride at 185°C gives an exothermic decomposition with rapid evolution of gas [Chem Eng. News 42(8); 41 1964]. Mixing Azabenzene in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, nitric acid (70%), oleum, sulfuric acid (96%), or propiolactone [NFPA 1991]. The combination of iodine, Azabenzene, sulfur trioxide, and formamide developed a gas over pressurization after several months. This arose from the slow formation of sulfuric acid from external water, or from dehydration of the formamide to hydrogen cyanide. Ethylene oxide and SO2 can react violently in Azabenzene solution with pressurization if ethylene oxide is in excess (Nolan, 1983, Case History 51).
Hazard
Flammable, dangerous fire risk, explosive limits in air 1.8–12.4%. Toxic by ingestion and inhalation. Skin irritant, liver and kidney damage. Questionable carcinogen.
Health Hazard
The acute toxicity of pyridine is low. Inhalation causes irritation of the respiratory
system and may affect the central nervous system, causing headache, nausea,
vomiting, dizziness, and nervousness. Pyridine irritates the eyes and skin and is
readily absorbed, leading to systemic effects. Ingestion of pyridine can result in liver
and kidney damage. Pyridine causes olfactory fatigue, and its odor does not provide
adequate warning of the presence of harmful concentrations.
Pyridine has not been found to be carcinogenic or to show reproductive or
developmental toxicity in humans. Chronic exposure to pyridine can result in
damage to the liver, kidneys, and central nervous system.
Health Hazard
The toxic effects of pyridine include headache,dizziness, nervousness, nausea, insomnia,frequent urination, and abdominal pain.The symptoms were transient, occurred inpeople from subacute exposure to pyridinevapors at about 125 ppm for 4 hours a dayfor 1–2 weeks (Reinhardt and Brittelli 1981).The target organs to pyridine toxicity are thecentral nervous system, liver, kidneys, gastrointestinaltract, and skin.
The routes ofexposure are inhalation of vapors, and ingestionand absorption of the liquid throughthe skin. Serious health hazards may arisefrom chronic inhalation, which may causekidney and liver damage, and stimulationof bone marrow to increase the productionof blood platelets. Low-level exposureto 10 ppm may produce chronic poisoningeffects on the central nervous system. Ingestionof the liquid may produce the samesymptoms as those stated above. Skin contactcan cause dermatitis. Vapor is an irritantto the eyes, nose, and lungs. Because of itsstrong disagreeable odor, there is always asufficient warning against any overexposure.A concentration of 10 ppm is objectionableto humans.
LCLO value, inhalation (rats): 4000 ppm/4 h
LD50 value, oral (mice): 1500 mg/kg.
Huh and coworkers (1986) have investigatedthe effect of glycyrrhetinic acid on pyridine toxicity in mice. Pretreatmentwith glycyrrhetinic acid decreaseddepression of the central nervous system andmortality in animals induced by pyridine.Such pretreatment markedly decreased theactivity of the enzyme serum transaminase, and increased the activity of hepaticmicrosomal aniline hydroxylase [9012-90-0], a pyridine- metabolizing enzyme.
Flammability and Explosibility
Pyridine is a highly flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance and "flash back." Pyridine vapor forms explosive mixtures with air at concentrations of 1.8 to 12.4% (by volume). Carbon dioxide or dry chemical extinguishers should be used for pyridine fires.
Industrial uses
Pyridine is a good solvent for a large number of compounds, both organic and inorganic (Windholz et al 1983). About 50% of pyridine used in the U.S. is for the production of agricultural chemicals, such as the herbicides paraquat, diquat and triclopyr and the insecticide chlorpyrifos. Other uses are in the production of piperidine; the manufacture of pharmaceuticals, such as steroids, vitamins and antihistamines; and as a solvent. Solvent uses are found in both the pharmaceutical and polycarbonate resin industries. It is particularly useful as a solvent in processes where HC1 is evolved (Santodonato et al 1985). Minor uses for pyridine are for the denaturation of alcohol and antifreeze mixtures, as a dyeing assistant in textiles and as a flavoring agent (Jori et al 1983; Furia 1968; HSDB 1988).
Contact allergens
Pyridine (unsubstituted pyridine) and its derivative (substituted pyridines) are widely used in chemistry. Pyridine is a solvent used for many organic compounds and anhydrous metallic salt chemicals. Contained in Karl Fischer reagent, it induced contact dermatitis in a laboratory technician. No cross-sensitivity is observed between those different substances.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion, skin contact, intravenous, and subcutaneous routes. Mildly toxic by inhalation. A skin and severe eye irritant. Mutation data reported. Can cause central nervous system depression, gastrointestinal upset, and liver and kidney damage. A flammable liquid and dangerous fire hazard when exposed to heat, flame, or oxidizers. Severe explosion hazard in the form of vapor when exposed to flame or spark. Reacts violently with chlorosulfonic acid, chromium trioxide, dinitrogen tetraoxide, HNO3, oleum, perchromates, ppropiolactone, AgClO4, H2SO4. Incandescent reaction with fluorine. Reacts to form pyrophoric or explosive products with bromine trifluoride, trifluoromethyl hypofluorite. Mixtures with formamide + iodine + sulfur trioxide are storage hazards, releasing carbon dioxide and sulfuric acid. Incompatible with oxidizing materials. Reacts with maleic anhydride (above 150°C) evolving carbon dioxide. To fight fire, use alcohol foam. When heated to decomposition it emits highly toxic fumes of NOx.
Potential Exposure
Pyridine is used as a solvent in the chemical industry and as a denaturant for ethyl alco- hol; as an intermediate in the production of pesticides; in pharmaceuticals; in the manufacture of paints, explosives, dyestuffs, rubber, vitamins, sulfa drugs; and disinfectants.
Carcinogenicity
Pyridine was not carcinogenic in
several chronic subcutaneous studies.
F344 rats were given pyridine orally in drinking water at
doses of 0, 7, 14, or 33 mg/kg for 2 years. The top dose
produced a decrease in body weights and water consumption.
Increased renal tubular adenoma or carcinoma and tubular
hyperplasia were observed in males at 33 mg/kg. Increased
mononuclear cell leukemia was observed in females at 14
and 33 mg/kg, which was considered equivocal in terms of
the relationship to pyridine exposure, since this is a common
finding in this strain of rat. Concentration-related nonneoplastic
change in the liver was seen at 33 mg/kg. Male
Wistar rats were similarly treated with doses of 0, 8, 17, or
36 mg/kg for 2 years. Decreased survival and body weights
were seen at 17 and 36 mg/kg. Increased testicular cell
adenomas were seen at 36 mg/kg. No changes in survival
or neoplasm rates in other tissues, including the kidney, were
reported although increased nephropathy and hepatic centrilobular
degeneration/necrosis was observed in some pyridine-
treated rats.
Source
Pyridine occurs naturally in potatoes, anabasis, henbane leaves, peppermint (0 to 1 ppb), tea leaves, and tobacco leaves (Duke, 1992). Identified as one of 140 volatile constituents in used soybean oils collected from a processing plant that fried various beef, chicken, and veal products (Takeoka et al., 1996).
Environmental Fate
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 1.31 g/g which is 58.7%
of the ThOD value of 2.23 g/g. A Nocardia sp. isolated from soil was capable of transforming
pyridine, in the presence of semicarbazide, into an intermediate product identified as succinic acid
semialdehyde (Shukla and Kaul, 1986). 1,4-Dihydropyridine, glutaric dialdehyde, glutaric acid
semialdehyde, and glutaric acid were identified as intermediate products when pyridine was
degraded by Nocardia strain Z1 (Watson and Cain, 1975).
Photolytic. Irradiation of an aqueous solution at 50 °C for 24 h resulted in a 23.06% yield of
carbon dioxide (Knoevenagel and Himmelreich, 1976).
Chemical/Physical. The gas-phase reaction of ozone with pyridine in synthetic air at 23 °C
yielded a nitrated salt having the formula: [C6H5NH]+NO3
- (Atkinson et al., 1987). Ozonation of
pyridine in aqueous solutions at 25 °C was studied with and without the addition of tert-butyl
alcohol (20 mM) as a radical scavenger. With tert-butyl alcohol, ozonation of pyridine yielded
mainly pyridine N-oxide (80% yield), which was very stable towards ozone. Without tert-butyl
alcohol, the heterocyclic ring is rapidly cleaved forming ammonia, nitrate, and the amidic
compound N-formyl oxamic acid (Andreozzi et al., 1991).
Metabolism
Pyridine is absorbed through the gastrointestinal tract, skin and lungs and is eliminated via the urine, feces, skin and lungs, both as metabolites and as the parent compound (Jori et al 1983). Uptake by tissues increases with dose and the elimination is biphasic in nature (Zharikov and Titov 1982; HSDB 1988). Elimination is rapid and there appears to be no tissue accumulation (Jori et al 1983). The observation by His (1887) of the urinary excretion of Af-methylpyridine by pyridine-dosed animals was the first example of Af-methylation. Known urinary metabolites of pyridine in mammals now include pyridine N-oxide, N-methyl pyridine, 4-pyridone, 2-pyridone and 3-hydroxypyridine. Some metabolites still remain to be identified (Damani et al 1982). The relative amounts of the metabolites are highly dependent on the species and dose (Gorrod and Damani 1980). For example, the rat has been shown to excrete 70% of a 1 mg/kg dose in the urine in the first 24 h after dosing, but that figure drops to only 5.8% for a 500 mg/kg dose (D'Souza et al 1980). Although urinary excretion of pyridine and its metabolites appears to be a major route for elimination, non-urinary excretion has not been extensively studied (Santodonato et al 1985). In rabbits, the pyridine N-methyltransferase activity has been shown to be highest in lung cytosol and it has been found to utilize 5-adenosyl methionine as the methyl donor (Damani et al 1986). This pathway is saturable in both the rat and the guinea pig (D'Souza et al 1980). The product of this reaction, N-methyl pyridine, is less chronically toxic but more acutely toxic than pyridine (Williams 1959). Pyridine N-oxide is produced by the cytochrome P-450 system and the activity is induced by phenobarbital or pyridine pretreatment but not by 3-methylcholanthrene (Gorrod and Damani 1979; Kaul and Novak 1987). In the rabbit, the alcohol-inducible (and pyridine inducible) P-450 LM3A appears to be the low Km isozyme which catalyzes pyridine Af-oxide production (Kim and Novak 1989). The N-oxidation of pyridine may represent a pathway for bioactivation (Santodonato et al 1985) and this pathway becomes more important as the pyridine dose is increased (Damani et al 1982).
storage
Pyridine should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.
Shipping
UN1992 Flammable liquids, toxic, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous mate- rials, Technical Name Required.
Purification Methods
Likely impurities are H2O and amines such as the picolines and lutidines. Pyridine is hygroscopic and is miscible with H2O and organic solvents. It can be dried with solid KOH, NaOH, CaO, BaO or sodium, followed by fractional distillation. Other methods of drying include standing with Linde type 4A molecular sieves, CaH2 or LiAlH4, azeotropic distillation of the H2O with toluene or *benzene, or treated with phenylmagnesium bromide in ether, followed by evaporation of the ether and distillation of the pyridine. A recommended [Lindauer Mukherjee Pure Appl Chem 27 267 1971] method dries pyridine over solid KOH (20g/Kg) for 2weeks and fractionally distils the supernatant over Linde type 5A molecular sieves and solid KOH. The product is stored under CO2-free nitrogen. Pyridine can be stored in contact with BaO, CaH2 or molecular sieves. Non-basic materials can be removed by steam distilling a solution containing 1.2 equivalents of 20% H2SO4 or 17% HCl until about 10% of the base has been carried over along with the non-basic impurities. The residue is then made alkaline, and the base is separated, dried with NaOH and fractionally distilled. Alternatively, pyridine can be treated with oxidising agents. Thus pyridine (800mL) has been stirred for 24hours with a mixture of ceric sulfate (20g) and anhydrous K2CO3 (15g), then filtered and fractionally distilled. Hurd and Simon [J Am Chem Soc 84 4519 1962] stirred pyridine (135mL), water (2.5L) and KMnO4 (90g) for 2hours at 100o, then stood for 15hours before filtering off the precipitated manganese oxides. Addition of solid KOH (ca 500g) caused pyridine to separate. It was decanted, refluxed with CaO for 3hours and distilled. Separation of pyridine from some of its homologues can be achieved by crystallisation of the oxalates. Pyridine is precipitated as its oxalate by adding it to the stirred solution of oxalic acid in acetone. The precipitate is filtered, washed with cold acetone, and pyridine is regenerated and isolated. Other methods are based on complex formation with ZnCl2 or HgCl2.
Incompatibilities
Violent reaction with strong oxidizers; strong acids; chlorosulfonic acid; maleic anhydride; oleum iodine.
Waste Disposal
Controlled incineration whereby nitrogen oxides are removed from the effluent gas by scrubber, catalytic or thermal devices .
Pyridine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Ningxia Jinhua Chemical Co.,Ltd | 025-52279164 | info@nxjhchem.com | China | 79 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hubei Langyou International Trading Co., Ltd | +8618874586545 | linda@xrdchem.cn | China | 197 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Anhui Royal Chemical Co., Ltd. | +86-25-86655873 +8613962173137 | marketing@royal-chem.com | China | 142 | 55 |
Related articles
- What are the effects of Pyridine on human health and the environment?
- Pyridine is a flammable, colourless liquid, six-membered heterocyclic compound with an unpleasant odour. Pyridine can cause fi....
- Jan 12,2024
- What is Pyridine?
- Pyridine is a heterocyclic compound with a chemical formula C5H5N.
- Nov 9,2022
- Pyridine: Uses & Synthesis
- Pyridine can also be used as a denaturant, dye assistant, and starting material for the synthesis of a series of products, inc....
- Nov 11,2021
View Lastest Price from Pyridine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Pyridine
110-86-1
|
US $10.00-1.00 / kg | 1kg | 99% | 150tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-26 | Pyridine
110-86-1
|
US $30.00-22.00 / kg | 1kg | 99% | 20Tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-17 | Pyridine
110-86-1
|
US $1.10 / g | 1g | 99.0% min | 100 tons min | Shaanxi Dideu Medichem Co. Ltd |
110-86-1(Pyridine)Related Search:
1of4