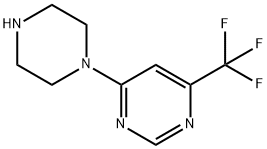
4-(1-PIPERAZINYL)-6-(TRIFLUOROMETHYL)PYRIMIDINE synthesis
- Product Name:4-(1-PIPERAZINYL)-6-(TRIFLUOROMETHYL)PYRIMIDINE
- CAS Number:845616-55-9
- Molecular formula:C9H11F3N4
- Molecular Weight:232.21

110-85-0
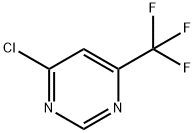
37552-81-1

845616-55-9
The general procedure for the synthesis of 4-(1-piperazinyl)-6-(trifluoromethyl)pyrimidine from piperazine and 4-chloro-6-trifluoromethylpyrimidine was as follows: 4-chloro-6-(trifluoromethyl)pyrimidine (1.0 g, 5.48 mmol), piperazine (2.36 g, 27.4 mmol) and triethylamine (2.29 mL, 16.4 mmol) were dissolved in N,N-dimethylformamide (DMF, 20 mL) in N,N-dimethylformamide (DMF, 20 mL) and the reaction was stirred at 100°C for 5 hours. After completion of the reaction, the reaction solution was diluted with water and extracted three times with ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by fast column chromatography (eluent: 10% methanol/5% triethylamine/ethyl acetate) to afford the target compound 4-(1-piperazine)-6-(trifluoromethyl)pyrimidine (720 mg, 56.6%). It was analyzed by liquid chromatography-mass spectrometry (LCMS), calculated value C9H12F3N4 [M+H]+: 233.1, measured value: 233.1.

110-85-0
587 suppliers
$5.00/5G

37552-81-1
192 suppliers
$14.00/1g

845616-55-9
24 suppliers
$70.00/100mg
Yield:845616-55-9 56.6%
Reaction Conditions:
with triethylamine in DMF (N,N-dimethyl-formamide) at 100; for 5 h;
Steps:
28.B
A solution of 4-chloro-6-(trifluoromethyl)pyrimidine (1.0 g, 5.48 mmol), piperazine (2.36 g, 27.4 mmol), and triethylamine (2.29 mL, 16.4 mmol) in DMF (20 mL) was stirred at 100° C. for 5 h. The reaction solution was diluted with water and extracted with ethyl acetate three times, dried with sodium sulfate, filtered, and concentrated in vacuo. The crude residue was purified by flash column chromatography (10% MeOH/5% Et3N/EtOAc) to yield the desired product (720 mg, 56.6%). LCMS calculated for C9H12F3N4: (M+1) 233.1; found 233.1.
References:
US2006/4018,2006,A1 Location in patent:Page/Page column 40