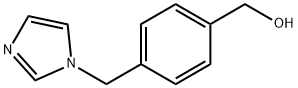
[4-(1H-IMIDAZOL-1-YLMETHYL)PHENYL]METHANOL synthesis
- Product Name:[4-(1H-IMIDAZOL-1-YLMETHYL)PHENYL]METHANOL
- CAS Number:103573-92-8
- Molecular formula:C11H12N2O
- Molecular Weight:188.23

288-32-4
1041 suppliers
$5.00/25g
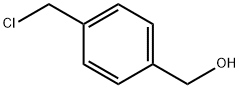
16473-35-1
131 suppliers
$16.00/250mg
![[4-(1H-IMIDAZOL-1-YLMETHYL)PHENYL]METHANOL](/CAS/GIF/103573-92-8.gif)
103573-92-8
20 suppliers
$110.00/250mg
Yield:103573-92-8 96%
Reaction Conditions:
Stage #1: 1H-imidazolewith potassium hydroxide in acetonitrile at 20; for 1 h;
Stage #2: 4-(hydroxymethyl)benzyl chloride in acetonitrile at 90; for 16 h;
Steps:
5 [4-(1H-Imidazol- 1-yl)methylphenyl]methanol (2).
A two-neck round-bottom flask fitted with a dropping funnel and reflux condenser was filled with imidazole (871 mg, 12.8 mmol) and powdered KOH (1.16 g, 16.6 mmol). Acetonitrile (70 mL) was added to the flask and the mixture was stirred at room temperature over 1 hour. The dropping funnel was then charged with a solution of benzyl chloride 1 (2.00 g, 12.8 mmol) in acetonitrile (57 mL) that was addeddropwise to the stirring mixture. Upon complete addition, the reaction mixture was stirred at reflux over 16 hours, then cooled to room temperature and concentrated under reduced pressure. The resultant solids were dissolved in chloroform (20 mL) and washed with water (20 mL). The aqueous layer was then extracted with ethyl acetate (2 x 20 mL). The combined organic layers were dried over Na2504, filtered and concentrated under reduced pressure to obtain benzylalcohol 2 as a yellow oil (2.31 g, 12.3 mmol, 96%). ‘H NMR (400 MHz, CDC13) 7.42 (s, 1H, ImH), 7.33 (d, J = 8.0 Hz, 2H, PhH), 7.08 (d, J = 8.0 Hz, 2H, PhH), 6.97 (t, J = 1.0 Hz, 1H, ImH), 6.85 (d, J = 1.0 Hz, 1H, 1mB), 5.04 (s, 1H, NCH2), 4.66 (s, 1H, OCH2); ‘3C NMR (101 MHz, CDC13) 141.4, 137.4, 135.3, 129.7, 127.5, 119.3, 64.6, 50.6.
References:
WO2019/4940,2019,A1 Location in patent:Page/Page column 34
56643-80-2
0 suppliers
inquiry
![[4-(1H-IMIDAZOL-1-YLMETHYL)PHENYL]METHANOL](/CAS/GIF/103573-92-8.gif)
103573-92-8
20 suppliers
$110.00/250mg
1201-90-7
91 suppliers
$11.00/1g
![[4-(1H-IMIDAZOL-1-YLMETHYL)PHENYL]METHANOL](/CAS/GIF/103573-92-8.gif)
103573-92-8
20 suppliers
$110.00/250mg
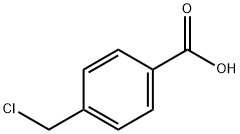
1642-81-5
391 suppliers
$17.00/5g
![[4-(1H-IMIDAZOL-1-YLMETHYL)PHENYL]METHANOL](/CAS/GIF/103573-92-8.gif)
103573-92-8
20 suppliers
$110.00/250mg