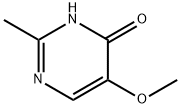
4(1H)-Pyrimidinone, 5-methoxy-2-methyl- (9CI) synthesis
- Product Name:4(1H)-Pyrimidinone, 5-methoxy-2-methyl- (9CI)
- CAS Number:698-35-1
- Molecular formula:C6H8N2O2
- Molecular Weight:140.14
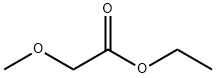
3938-96-3
145 suppliers
$10.00/5g
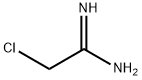
20846-52-0
37 suppliers
inquiry
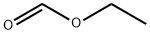
109-94-4
503 suppliers
$10.00/10g

698-35-1
22 suppliers
$200.00/50mg
Yield:698-35-1 56.2%
Reaction Conditions:
with sodium in diethyl ether;ethanol;
Steps:
2 4-hydroxy-2-methyl-5-methoxypyrimidine
Example 2 4-hydroxy-2-methyl-5-methoxypyrimidine A modified form of the Maccoss Malcolm EP 330263 process was used. To a suspension of sodium (4.88 g 0.212 at-gr) in 300 ml of ethyl ether was added dropwise over 1 hour a mixture of 25 g (0.212 mol) of ethylmethoxyacetate and 23.5 g (0.23 mol) of ethyl formate. At the end of the addition, the reaction was refluxed under a N2 atmosphere for 2 hours and thereafter at room temperature overnight. The solvent was removed by decantation and the residue was washed with 3 x 100 ml of ethyl ether. The residue was dissolved in 250 ml of ethanol and 20 g (0.212 mol) acetamidine chloride were added. The mixture was refluxed for 6 hours. It was cooled to room temperature and the salts were filtered. The filter liquors were concentrated to dryness and chromatographed on silica gel, eluding with dichloromethane/ethanol 7/3, yielding 16.7 g of a white solid (56.2%).
References:
EP714896,1996,A1