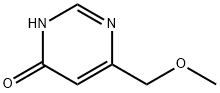
4(1H)-Pyrimidinone, 6-(methoxymethyl)- (9CI) synthesis
- Product Name:4(1H)-Pyrimidinone, 6-(methoxymethyl)- (9CI)
- CAS Number:3122-78-9
- Molecular formula:C6H8N2O2
- Molecular Weight:140.14
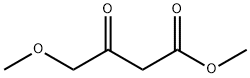
41051-15-4
303 suppliers
$6.00/5g
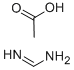
3473-63-0
515 suppliers
$5.00/25g

3122-78-9
20 suppliers
$18.00/100mg
Yield: 37.1%
Reaction Conditions:
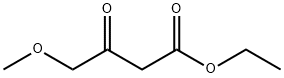
Stage #1:4-methoxyacetoacetic acid methylester;formamidine acetic acid with methanol;sodium methylate for 18 h;Reflux;
Stage #2: with hydrogenchloride in water; pH=7
Steps:
218.A
Step A: 6-(Methoxymethyl)pyrimidin-4-ol To a solution of methyl 4-methoxy-3-oxobutanoate (3.54 mL, 26.5 mmol) in methanol (30 ml) was added formamidine acetate (3.07 g, 29.2 mmol) and sodium methoxide (13 mL, 58.4 mmol). The mixture was then heated to reflux for 18 hours and cooled to ambient temperature, then concentrated. The residue was taken up in water and the pH adjusted to 7 with 1N HCl. The aqueous mixture was extracted with chloroform. The combined organic layers were dried over magnesium sulfate, filtered and concentrated to afford 6-(methoxymethyl)pyrimidin-4-ol (1.38 g, 9.85 mmol, 37.1% yield). MS (LC/MS) R.T.=0.19; [M+H]+=141.20.
References:
Bristol-Myers Squibb Company US2009/270405, 2009, A1 Location in patent:Page/Page column 126

41051-15-4
303 suppliers
$6.00/5g
6313-33-3
201 suppliers
$9.00/1g

3122-78-9
20 suppliers
$18.00/100mg
66762-68-3
76 suppliers
$36.00/1g

3122-78-9
20 suppliers
$18.00/100mg
