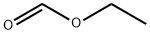4(1H)-Quinolinone, 7-Methoxy-6-(phenylMethoxy)- synthesis
- Product Name:4(1H)-Quinolinone, 7-Methoxy-6-(phenylMethoxy)-
- CAS Number:516526-42-4
- Molecular formula:C17H15NO3
- Molecular Weight:281.31
Yield: 85%
Reaction Conditions:
with sodium methylate in tetrahydrofuran at 20; for 1.5 h;
Steps:
13 Preparation Example 13: Production of 6-benzyloxy-7-methoxy-4-quinolone (Starting compound 17)
2-Amino-5-benzyloxy-4-methoxyacetophenone (13.1 g), tetrahydrofuran (anhydrous) (200 ml), and sodium methoxide (5 eq, 13.1 g) were added, and the mixture was stirred at room temperature for 30 min. Ethyl formate (5 eq, 19.4 ml) was added thereto, and the mixture was further stirred at room temperature for one hr. Water was added thereto, and the mixture was stirred at room temperature for one hr, followed by concentration under the reduced pressure. The concentrate was rendered weakly acidic by the addition of 10% aqueous hydrochloric acid. Chloroform was added thereto, the mixture was extracted with chloroform, and the extract was washed with saturated brine, was dried over sodium sulfate, and the solvent was then removed by evaporation under the reduced pressure. The crude thus obtained was purified by chromatography on silica gel using chloroform/methanol for development to give the title compound (11.5 g, yield 85%). 1H-NMR (CDCl3, 400 MHz): δ 3.97 (s, 3H), 5.19 (s, 2H), 6.28 (d, J = 7.3 Hz, 1H), 7.02 (s, 1H), 7.29 - 7.41 (m, 3H), 7.47 - 7.51 (m, 2H), 7.71 (s, 1H), 7.86 (d, J = 7.3 Hz, 1H)
References:
KIRIN BEER KABUSHIKI KAISHA EP1447405, 2004, A1 Location in patent:Page 29
![Ethanone,1-[2-amino-4-methoxy-5-(phenylmethoxy)phenyl]-](/CAS/GIF/516526-41-3.gif)