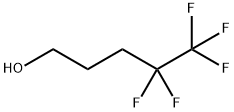
4,4,5,5,5-Pentafluoro-1-pentanol synthesis
- Product Name:4,4,5,5,5-Pentafluoro-1-pentanol
- CAS Number:148043-73-6
- Molecular formula:C5H7F5O
- Molecular Weight:178.1
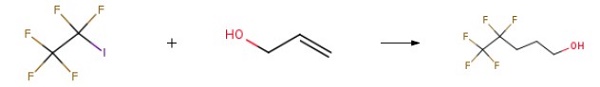
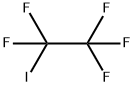
354-64-3
175 suppliers
$45.00/5G

107-18-6
0 suppliers
$24.60/100ml

148043-73-6
291 suppliers
$15.00/2g
Yield:148043-73-6 90.4%
Reaction Conditions:
with 6,6'-dimethyl-2,2'-bipyridine;tris(2,4-pentanedionato)iron(III);chlorotriisopropylsilane;Cs2CO3 in 1,2-dichloro-ethane at 80; for 24.5 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
1-5 Example 4
Under inert gas atmosphere, iron acetylacetonate (17.7 g, 50 mmol), 6,6'-dimethyl-2,2'-bipyridine (18.4 g, 0.10 mol) and 1,2-dichloroethane ( 500ml) was added to the 2.5L reactor, reacted at room temperature for 0.5 hour; added allyl alcohol (58.0g, 1.0mol), cesium carbonate (326g, 1.0mol), pentafluoroiodoethane (492g, 2.0mol) successively ), triisopropylsilane (316g, 2.0mol), warmed up to 80 and reacted for 24 hours. Sampling and detection confirmed that the reaction of allyl alcohol was complete, stopped the reaction, and cooled to room temperature.The excess pentafluoroiodoethane was recovered, nitrogen was introduced into the system, 1000 ml of 2M hydrochloric acid was added to quench the reaction, washed, separated to obtain an organic phase, distilled to remove the solvent, and rectified to obtain 4,4,5,5,5-pentafluoro Amyl alcohol (161 g, yield 90.4%, purity: 99.4%).
References:
CN114853568,2022,A Location in patent:Paragraph 0049-0058
757-06-2
22 suppliers
inquiry

148043-73-6
291 suppliers
$15.00/2g
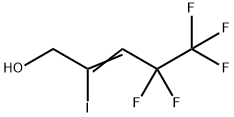
172032-07-4
0 suppliers
inquiry

148043-73-6
291 suppliers
$15.00/2g
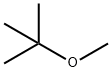
1634-04-4
692 suppliers
$14.00/100g

757-06-2
22 suppliers
inquiry

148043-73-6
291 suppliers
$15.00/2g
