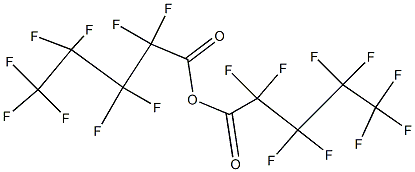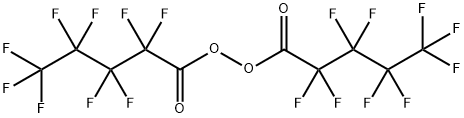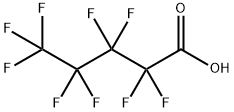
Perfluoropentanoic acid synthesis
- Product Name:Perfluoropentanoic acid
- CAS Number:2706-90-3
- Molecular formula:C5HF9O2
- Molecular Weight:264.05
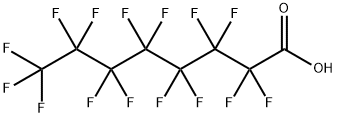
335-67-1
325 suppliers
$10.00/1 g
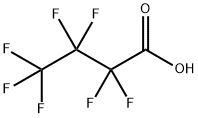
375-22-4
267 suppliers
$9.00/1g

2706-90-3
158 suppliers
$12.00/1g
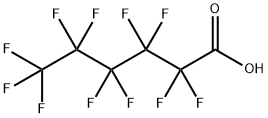
307-24-4
208 suppliers
$20.00/2 g
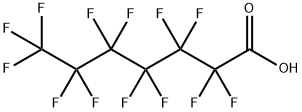
375-85-9
197 suppliers
$5.00/100mg
Yield:-
Reaction Conditions:
with sulfuric acid;LaNi0.8Sr0.2O3;iron(II) sulfate;sodium sulfate in water; pH=5 for 2.5 h;Catalytic behavior;Kinetics;Mechanism;Electrolysis;Reagent/catalyst;pH-value;
Steps:
A 0.05 mol/L Na2SO4 solution was used as the supporting electrolyte, and the pH was adjusted by H2SO4 and NaOH. The prepared GDE was used as the cathode, and graphite as the anode. The distance between the anode and cathode was 30 mm, the current density was 20 mA/cm2, and the aeration rate was 100 L/min. 1.0 mmol/L ferrous sulfate (FeSO4) was used to enhance catalytic activity. The PFOA solution with an initial concentration of 0.25 mmol/L was degraded in the reaction tank. During the electrolytic process, the solution was periodically sampled and analyzed. Batch experiments were conducted to explore the optimal ratio of La, Ni, and Sr, along with the influences of pH and initial concentration of PFOA on the performance of PFOA degradation.
References:
Chen, Hao;Chen, Yongyang;Dong, Xiaochun;Guo, Dan;Huang, Yixuan;Li, Shanping;Li, Yahui [Journal of Fluorine Chemistry,2021,vol. 242,art. no. 109700]
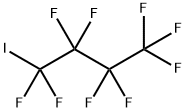
423-39-2
254 suppliers
$6.00/5g

124-38-9
134 suppliers
$175.00/23402

2706-90-3
158 suppliers
$12.00/1g

335-67-1
325 suppliers
$10.00/1 g

375-22-4
267 suppliers
$9.00/1g
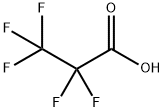
422-64-0
269 suppliers
$6.00/5g

2706-90-3
158 suppliers
$12.00/1g

307-24-4
208 suppliers
$20.00/2 g

375-85-9
197 suppliers
$5.00/100mg
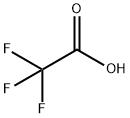
76-05-1
725 suppliers
$9.00/1g
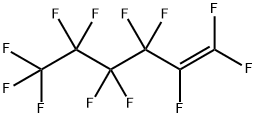
755-25-9
28 suppliers
$60.00/100mg

2706-90-3
158 suppliers
$12.00/1g