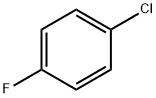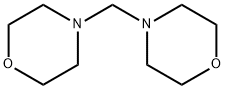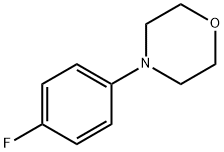
4-(4-FLUORO-PHENYL)-MORPHOLINE synthesis
- Product Name:4-(4-FLUORO-PHENYL)-MORPHOLINE
- CAS Number:4280-40-4
- Molecular formula:C10H12FNO
- Molecular Weight:181.21
Yield:4280-40-4 96%
Reaction Conditions:
with C19H14N4NiO2;potassium tert-butylate in 1,4-dioxane at 90; for 4 h;Inert atmosphere;Schlenk technique;Buchwald-Hartwig Coupling;
Steps:
General procedure for the Buchwald-Hartwig reaction
General procedure: Under an N2atmosphere, KOtBu (1.3 mmol), complex 1 (1 mol%),dioxane (2 ml), amines (1.3 mmol) and aryl chlorides (1.0 mmol)were successively added into a Schlenk tube. The mixture wasstirred vigorously at 90C for 4 h. Then the solvent was removedunder reduced pressure and the residue was purified by columnchromatography on silica gel (eluent:PE/EA = 15:1) to give the pureproducts. The reported yields are the average of two runs.The catalytic reactions have been given in Tables 4-7. The result-ing amines were identified by comparison of the1H and13C NMRdata with those previously reported (ESI).
References:
Nirmala, Muthukumaran;Prakash, Govindan;Ramachandran, Rangasamy;Viswanathamurthi, Periasamy;Malecki, Jan Grzegorz;Linert, Wolfgang [Journal of Molecular Catalysis A: Chemical,2015,vol. 397,p. 56 - 67]