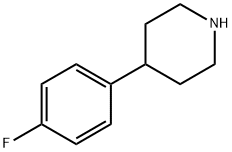
4-(4-FLUORO-PHENYL)-PIPERIDINE synthesis
- Product Name:4-(4-FLUORO-PHENYL)-PIPERIDINE
- CAS Number:37656-48-7
- Molecular formula:C11H14FN
- Molecular Weight:179.23
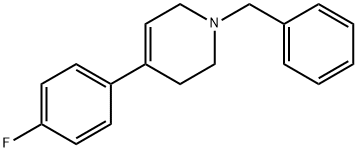
163630-89-5

37656-48-7
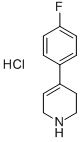
4-(4-Fluorophenyl)-1,2,3,6-tetrahydro-1-(phenylmethyl)pyridine (60 g, 225 mmol) was used as a raw material and dissolved in methanol (500 mL). To this solution, palladium/carbon hydroxide (20%, 5 g) was added and the reaction was shaken under hydrogen atmosphere (50 psi) for 48 hours. Upon completion of the reaction, the mixture was filtered through a glass fiber mat and washed with methanol. The filtrate was concentrated under reduced pressure to remove the solvent. The residue was dissolved in ethyl acetate and a 1 M solution of ether hydrogen chloride (300 mL) was added. The resulting solid was collected and recrystallized from 2-propanol to give 4-(4-fluorophenyl)piperidine hydrochloride as a colorless solid (30.5 g, 63% yield). Mass spectral data: m/z (ES+) 180 (M+1). The resulting 4-(4-fluorophenyl)piperidine hydrochloride sample (1 g, 4.64 mmol) was suspended in ethyl acetate and washed with saturated aqueous sodium carbonate. The organic layer was dried with anhydrous magnesium sulfate and the solvent was evaporated under reduced pressure to give the title compound 4-(4-fluorophenyl)piperidine as a colorless oil (825 mg, 99% yield). Mass spectral data: m/z (ES+) 180 (M+1).

163630-89-5
9 suppliers
inquiry

37656-48-7
109 suppliers
$22.00/250mg
Yield:37656-48-7 94%
Reaction Conditions:
with H2;Pd(OH)2 in methanol;
Steps:
b b.
b. 4-(4-fluoro)-phenyl-piperidine To a solution of 1-benzyl-4-(4-fluoro-phenyl)-1,2,3,6-tetrahydro-pyridine (45.0 mmol, 12.0 g) in 100 mL MeOH was added 1.0 g of Pd(OH)2 and the resulting suspension was hydrogenated under 200 psi of H2 in a stainless steel bomb for two days. The suspension was passed through a pad of celite and the filtrate was concentrated in vacuo to obtain 4-(4-fluoro)-phenyl-piperidine (7.5 g, 94%) as a viscous oil.
References:
US6218390,2001,B1
183950-95-0
3 suppliers
inquiry

37656-48-7
109 suppliers
$22.00/250mg
1978-61-6
56 suppliers
$45.00/50mg

37656-48-7
109 suppliers
$22.00/250mg
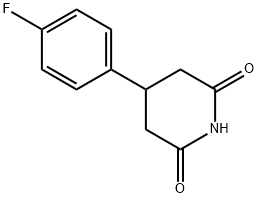
163631-01-4
15 suppliers
inquiry

37656-48-7
109 suppliers
$22.00/250mg
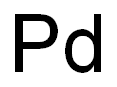
7440-05-3
503 suppliers
$17.69/1G

1978-61-6
56 suppliers
$45.00/50mg

37656-48-7
109 suppliers
$22.00/250mg