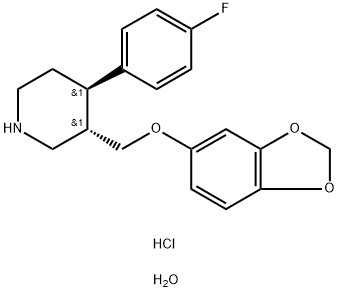
PAROXETINE-D4 HCL synthesis
- Product Name:PAROXETINE-D4 HCL
- CAS Number:110429-35-1
- Molecular formula:C19H23ClFNO4
- Molecular Weight:383.84
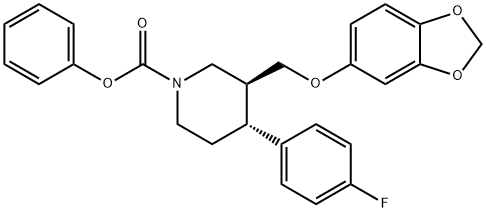
253768-88-6
25 suppliers
inquiry

110429-35-1
304 suppliers
$23.00/100mg
Yield:110429-35-1 97%
Reaction Conditions:
Stage #1: (3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine-1-carboxylic acid phenyl esterwith potassium hydroxide in sulfolane;toluene at 80 - 85; for 4 h;
Stage #2: with water in sulfolane;toluene at 30 - 35;
Stage #3: with hydrogenchloride in water;toluene at 20 - 25; for 2 h;Product distribution / selectivity;
Steps:
6
Example 6:; Preparation of (-)-*r??s-4-(4-fluoropheny)-3-[[3,4-(methyIenedioxy)phenoxy] methyLJpiperidine, compound of formula I; A mixture of compound of formula II (1Og), toluene (50ml), sulfolane (10ml) and potassium hydroxide (6g) is heated to 80-850C for 4hrs, cooled to 30-350C and then quenched by addition of water (30ml) at 30-350C. The upper toluene layer was separated and the aqueous layer extracted with toluene (20ml). The combined toluene layer were washed successively with water and saturated aqueous sodium chloride solution, charcoalized and acidified with cone, hydrochloric acid. After stirring at 20-250C for 2hrs the product was filtered and washed with toluene followed by water. The product was dried to constant weight to yield paroxetine hydrochloride hemihydrate, as a white crystalline solid, 8. Ig (97% yield), purity 99.87% by HPLC.
References:
WO2007/15262,2007,A2 Location in patent:Page/Page column 18
![1-Piperidinecarboxylic acid, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, 1,1-dimethylethyl ester, (3S,4R)-](/CAS/20210305/GIF/200572-35-6.gif)
200572-35-6
0 suppliers
inquiry

110429-35-1
304 suppliers
$23.00/100mg
![1-Piperidinecarboxylic acid, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, phenylmethyl ester, (3S,4R)-](/CAS/20210305/GIF/246850-71-5.gif)
246850-71-5
0 suppliers
inquiry

110429-35-1
304 suppliers
$23.00/100mg
![1-Piperidinecarboxylic acid, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, 1,1-dimethylethyl ester, (3S,4R)-](/CAS/20210305/GIF/200572-35-6.gif)
200572-35-6
0 suppliers
inquiry

110429-35-1
304 suppliers
$23.00/100mg
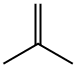
115-11-7
243 suppliers
$45.00/25g
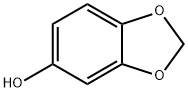
533-31-3
629 suppliers
$5.00/100mg

110429-35-1
304 suppliers
$23.00/100mg