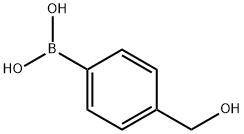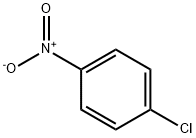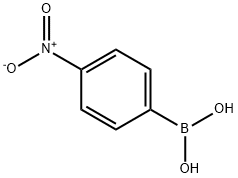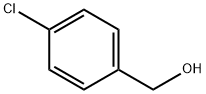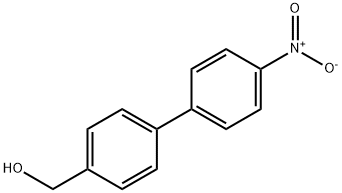
4-(4-Nitrophenyl)benzyl alcohol synthesis
- Product Name:4-(4-Nitrophenyl)benzyl alcohol
- CAS Number:62037-99-4
- Molecular formula:C13H11NO3
- Molecular Weight:229.23
Yield: 99%
Reaction Conditions:
with C37H44ClN2O3PPd;caesium carbonate in water at 100; for 12 h;Schlenk technique;Inert atmosphere;Suzuki Coupling;
Steps:
General procedure for Suzuki coupling
General procedure: In a Schlenk tube, a mixture of the required amount of catalyst, plus the aryl chloride (1.0 mmol), aryl boronic acid (1.5 mmol) and the selected base (2.0 mmol) in water was evacuated and charged with nitrogen. The reaction mixture was heated at 100°C for 12 h. After cooling, the mixture was extracted with CH2Cl2 and the extract was evaporated. The resulting residue was purified by flash chromatography on silica gel using a mixture of CH2Cl2/ethyl acetate (5/1) as eluent. The known products 5, 6a [17,18], 6b [22], 6c [23], 6e [24], 6g [19], 6h [25] and 7a [26] were characterized by comparison of data with those in the literature. The products 6d, 6f and 7b-h were new compounds and characterized by elemental analysis, MS, 1H and 13C NMR.
References:
Han, Xin;Li, Hong-Mei;Xu, Chen;Xiao, Zhi-Qiang;Wang, Zhi-Qiang;Fu, Wei-Jun;Hao, Xin-Qi;Song, Mao-Ping [Transition Metal Chemistry,2016,vol. 41,# 4,p. 403 - 411]
![4'-Nitro-[1,1'-biphenyl]-4-carboxaldehyde](/CAS/GIF/98648-23-8.gif)
98648-23-8
61 suppliers
$34.00/250mg

62037-99-4
24 suppliers
inquiry
720-75-2
123 suppliers
$34.00/5g

62037-99-4
24 suppliers
inquiry
1122-91-4
672 suppliers
$9.00/10g

62037-99-4
24 suppliers
inquiry