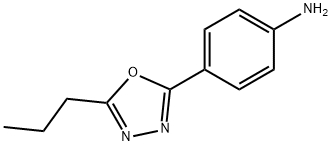
4-(5-Propyl-1,3,4-oxadiazol-2-yl)aniline synthesis
- Product Name:4-(5-Propyl-1,3,4-oxadiazol-2-yl)aniline
- CAS Number:1227954-86-0
- Molecular formula:C11H13N3O
- Molecular Weight:203.24

944661-22-7
7 suppliers
inquiry

1227954-86-0
9 suppliers
$96.00/250mg
Yield:1227954-86-0 98%
Reaction Conditions:
with sodium sulphide nonahydrate in 1,4-dioxane;lithium hydroxide monohydrate at 80; for 0.75 h;Inert atmosphere;
Steps:
10 2-(4′-Aminophenyl)-5-propyl-1,3,4-oxadiazole (10)
3.1.10
2-(4'-Aminophenyl)-5-propyl-1,3,4-oxadiazole (10)
A similar procedure
23
to that previously described for the preparation of 1 was followed using 3-propyl-5-(4'-nitrophenyl)-1,3,4-oxadiazole (66) (27 mg, 0.12 mmol) in 1,4-dioxane (1 mL) and sodium sulfide nonahydrate (67 mg, 0.28 mmol) in water (1 mL).
Purification by flash chromatography (hexane/ethyl acetate 1:1) afforded 10 as a pale brown solid (23 mg, 0.11 mmol, 98%). Rf = 0.15 (hexane/ethyl acetate, 1:1); mp 127-132 °C; 1H NMR (400 MHz; CDCl3) δH 1.05 (3H, t, J = 7.5 Hz, CH2CH2CH3), 1.86 (2H, m, CH2CH2CH3), 2.86 (2H, t, J = 7.5 Hz, CH2CH2CH3), 4.09 (2H, s, NH2), 6.72 (2H, d, J = 8.4 Hz, H-3', H-5') and 7.82 (2H, d, J = 8.4 Hz, H-2', H-6'); 13C NMR (100 MHz, CDCl3) δC 13.6 (CH3), 20.1 (CH2), 27.2 (CH2), 113.7 (C), 114.6 (CH), 128.4 (CH), 149.5 (C), 165.0 (C) and 165.8 (C); IR (νmax/cm-1) 826, 1172 (C-O), 1315 (C-N), 1498, 1569 (N-H), 1607, 1739, 2970, 3207, 3324 (N-H) and 3425; MS (ESI, 70 eV) m/z 204 (M+, 100%); Found (M+, 204.1131), C11H14N3O requires 204.1131.
References:
Conole, Daniel;Beck, Thorsten M.;Jay-Smith, Morgan;Tingle, Malcolm D.;Eason, Charles T.;Brimble, Margaret A.;Rennison, David [Bioorganic and medicinal chemistry]
122-04-3
39 suppliers
$15.00/1g

1227954-86-0
9 suppliers
$96.00/250mg
636-97-5
18 suppliers
$13.43/1gm:

1227954-86-0
9 suppliers
$96.00/250mg