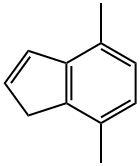
4,7-DIMETHYL-1H-INDENE synthesis
- Product Name:4,7-DIMETHYL-1H-INDENE
- CAS Number:6974-97-6
- Molecular formula:C11H12
- Molecular Weight:144.21
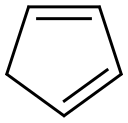
542-92-7
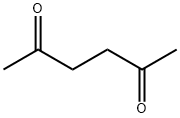
110-13-4

6974-97-6
The general procedure for the synthesis of 4,7-dimethyl-1H-indene from cyclopentadiene and 2,5-hexanedione was as follows: sodium filaments (426 mmol, 9.8 g) were pressed into a three-necked flask under argon protection, and about 100 mL of dry methanol was added slowly until the sodium filaments were completely dissolved. Subsequently, freshly distilled cyclopentadiene (260 mmol, 21.2 mL) and 2,5-hexanedione (170 mmol, 26.5 mL) were added, and the reaction mixture was stirred for 24 h, during which time bubbles were observed. Upon completion of the reaction, 50 mL of water was added to quench the reaction. After removal of the methanol solvent by steam distillation, the pH of the reaction solution was adjusted to neutral with dilute hydrochloric acid. The aqueous phase was extracted three times with 200 mL of petroleum ether, and the organic phases were combined, dried over anhydrous magnesium sulfate and filtered. The solvent was removed by rotary evaporation to give a dark brown liquid. The liquid was distilled under reduced pressure and the 76-80 °C fraction was collected at a pressure of 4 mmHg to give a final 21.5 g of yellow oily liquid product.
Yield: 87%
Reaction Conditions:
with sodium in methanol for 24 h;Inert atmosphere;
Steps:
2.1 Preparation of 4,7-dimethylindene
Preparation of 4,7-dimethylindeneUnder argon, the sodium wire was pressed into a three-necked flask(426 mmol, 9.8 g),Slowly add about 100mLDry methanol, until the sodium wire disappeared, adding the new system of cyclopentadiene (260mmol, 21.2mL) andCyclopentene2,5-hexanedione(170 mmol, 26.5 mL) and stirred for 24 h for bubbling.Add 50 mL of water to quench the reaction. After the methanol solvent was removed by steaming,With dilute hydrochloric acid to adjust the pH to neutral after the liquid,The aqueous phase was extracted with 200 mL of petroleum ether three times. The organic phases were combined, dried over anhydrous magnesium sulfate, filtered,Rotate the solvent to remove the dark brown liquid,This was subjected to distillation under reduced pressure, and a fraction of 76 to 80 ° C was collected at 4 mmHg,To give 21.5 g of a yellow oily liquid,
References:
East China University of Science and Technology;Ma, Haiyan;Li, Bo;Wang, Han;Huang, Jiling CN106565404, 2017, A Location in patent:Paragraph 0076; 0077; 0078

70839-99-5
2 suppliers
inquiry

6974-97-6
55 suppliers
$15.00/1g
104-15-4
764 suppliers
$133.00/500g

6974-97-6
55 suppliers
$15.00/1g
5037-60-5
39 suppliers
inquiry

6974-97-6
55 suppliers
$15.00/1g

7732-18-5
507 suppliers
$12.69/100ml

542-92-7
93 suppliers
inquiry

110-13-4
398 suppliers
$10.00/5g

6974-97-6
55 suppliers
$15.00/1g