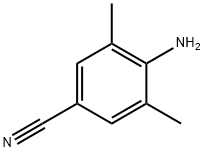
4-AMINO-3,5-DIMETHYL-BENZONITRILE synthesis
- Product Name:4-AMINO-3,5-DIMETHYL-BENZONITRILE
- CAS Number:74896-24-5
- Molecular formula:C9H10N2
- Molecular Weight:146.19
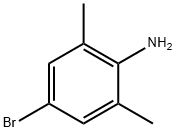
24596-19-8
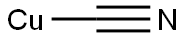
544-92-3

74896-24-5
GENERAL STEPS: A mixture of 4-bromo-2,6-dimethylaniline (1.90 g, 9.50 mmol) and cuprous(I) cyanide (1.71 g, 19.1 mmol) in N-methylpyrrolidone (NMP, 25 mL) was reacted with stirring at 160 °C overnight. Upon completion of the reaction, the mixture was cooled to room temperature. Subsequently, water (10 mL) and ammonium hydroxide solution (10 mL) were added to the reaction mixture and extracted with ethyl acetate (3 × 50 mL) to separate the products. The organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was purified by fast column chromatography (dichloromethane as eluent) to afford 4-amino-3,5-dimethylbenzonitrile 1.19 g (86% yield, 1.39 g theoretical yield). The product was analyzed by LCMS (Method B) with a retention time (RT) of 1.925 min and a molecular ion peak (MH+) of 147. 1H NMR (500 MHz, CDCl3) δ 7.22 (s, 2H), 2.18 (s, 6H).
Yield: 86%
Reaction Conditions:
in 1-methyl-pyrrolidin-2-one at 160;
Steps:
AN.1
The mixture of 4-bromo-2,6-dimethylaniline (1.90 g, 9.50 mmol) and copper (I) cyanide (1.71 g, 19.1 mmol) in NMP (25 mL) was stirred at 160° C. overnight. The reaction mixture was cooled to room temperature. Water (10 mL) and ammonium hydroxide (10 mL) were added and the product was extracted with ethyl acetate (2×50 mL). The product was purified by flash chromatography (DCM) to give 1.19 g (86%, theoretical yield 1.39 g). LCMS (method B) RT 1.925 min., MH+ 147. 1H NMR (500 MHz, CDCl3) δ 7.22 (s, 2H), 2.18 (s, 6H).
References:
Gerritz, Samuel;Shi, Shuhao;Zhu, Shirong US2006/287287, 2006, A1 Location in patent:Page/Page column 34
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

74896-24-5
96 suppliers
inquiry
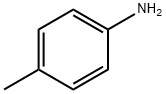
106-49-0
410 suppliers
$5.00/5 g
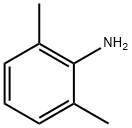
87-62-7
462 suppliers
$10.00/5 g

74896-24-5
96 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

74896-24-5
96 suppliers
inquiry