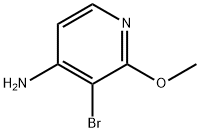
4-AMINO-3-BROMO-2-METHOXYPYRIDINE synthesis
- Product Name:4-AMINO-3-BROMO-2-METHOXYPYRIDINE
- CAS Number:215364-86-6
- Molecular formula:C6H7BrN2O
- Molecular Weight:203.04
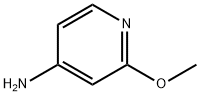
20265-39-8

215364-86-6
Synthesis of 3-bromo-2-methoxypyridin-4-amine (2): 2-Methoxypyridin-4-amine (1, 20.0 g, 161.2 mmol) was dissolved in dichloromethane (200 mL) at 0 °C. To this solution, N-bromosuccinimide (26.6 g, 161.2 mmol) was slowly added, followed by warming the reaction mixture to 30 °C and stirring for 30 min. Upon completion of the reaction, the reaction was quenched with ice-cold water (100 mL) and the reaction mixture was extracted with dichloromethane (300 mL). The organic layers were combined, washed sequentially with water and brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was ground with a solvent mixture of n-pentane and ether to give 3-bromo-2-methoxypyridin-4-amine (2) as a yellow solid. Yield: 30.0 g, 92% yield; MS (ESI) m/z 203.09 [M + 1]+.

20265-39-8
254 suppliers
$6.00/1g

215364-86-6
83 suppliers
$65.00/100mg
Yield: 96.6%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 25; for 4 h;
Steps:
A Step A.3-Bromo-2-methoxypyridin-4-amine.
To a solution of 2-methoxypyridin-4-amine (5.0 g, 40.28 mmol) in DCM (10 mL) was added NBS (7.17 g, 40.28 mmol) at 0°C. Then the reaction mixture was stirred at 25°C for 4 hrs. The solvent was removed under reduced pressure. The residue was purified by column chromatography (SiO2, Petroleum ether/EtOAc=5/1 to 2/1) to give the title compound (7.9 g, 38.91 mmol, 96.60% yield) as a yellow oil.1H NMR (400 MHz, CDCl3) d = 7.67 (d, J = 5.6 Hz, 1H), 6.26 (d, J = 5.6 Hz, 1H), 3.90 (s, 3H).
References:
JANSSEN BIOTECH, INC.;CISAR, Justin;KUDUK, Scott;WANG, Chao-yuan;SIMONNET, Yvan Rene Ferdinand;KEOHANE, Colleen Elizabeth WO2020/144638, 2020, A1 Location in patent:Page/Page column 86

67-56-1
790 suppliers
$7.29/5ml-f
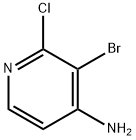
215364-85-5
140 suppliers
$11.00/250mg

215364-86-6
83 suppliers
$65.00/100mg
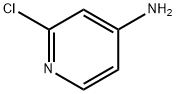
14432-12-3
540 suppliers
$5.00/5g

215364-86-6
83 suppliers
$65.00/100mg