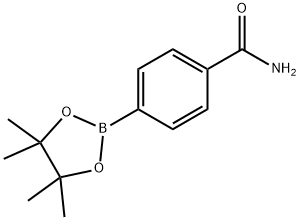
4-AMINOCARBONYLPHENYLBORONIC ACID, PINACOL ESTER synthesis
- Product Name:4-AMINOCARBONYLPHENYLBORONIC ACID, PINACOL ESTER
- CAS Number:179117-44-3
- Molecular formula:C13H18BNO3
- Molecular Weight:247.1

73183-34-3
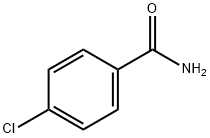
619-56-7

179117-44-3
Under argon protection, 1.4 mg (0.005 mmol) of dichlorobis(trimethylphosphine)nickel (NiCl2(PMe3)2), 77.4 mg (0.5 mmol) of 4-chlorobenzamide, 152 mg (1.0 mmol) of cesium fluoride (CsF), 140 mg (0.55 mmol) of bis(pinacolato)bis(boronic acid) ( B2pin2), 180 mg (1.05 mmol) trimethyl (2,2,5,5-tetrafluoroethoxy) silane (TMSEF), and 0.5 mL 1,4-dioxane. The reaction vessel was sealed and the mixture was stirred and reacted at 100°C for 12 hours. Upon completion of the reaction, the reaction vessel was cooled to room temperature and the reaction was quenched by the addition of 1 mL of saturated aqueous ammonium chloride. The mixture was extracted three times with 8 mL of ethyl acetate and the organic phases were combined. The solvent was removed by distillation under reduced pressure and the residue was purified by silica gel column chromatography (eluent gradient: hexane/chloroform/ethyl acetate = 4:1:0 to 4:1:1) to afford 92 mg (75% yield) of the target product 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide as a white solid.
180516-87-4
244 suppliers
$8.00/1g

179117-44-3
83 suppliers
$8.00/250mg
Yield: 45%
Reaction Conditions:
with ammonia;benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in dichloromethane at 20; for 3 h;
Steps:
62.1
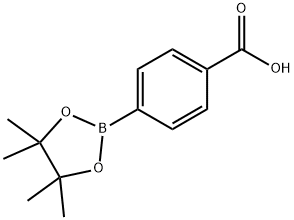
Step 1 4-(4,4,5,5-Metramethyl-1,3,2-dioxaborolan-2-yl)benzamide Procedure To a mixture of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoic acid (200 mg, 0.746 mmol), EDCI (214 mg, 1.12 mmol), HOBt (151 mg, 1.12 mmol) and Et3N (151 mg, 1.49 mmol) in DCM (20 mL) was bubbled ammonia until saturation. The mixture was stirred at room temperature for 3 h, then was filtered and the filtrate was concentrated to give residue which was purified by column chromatography (DCM:MeOH=50:1) to afford 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide (90 mg, 45%) as a yellow solid. LC-MS: 248 [M+H]+, tR=1.421 min.
References:
Hermann, Johannes Cornelius;Lowrie, JR., Lee Edwin;Lucas, Matthew C.;Luk, Kin-Chun Thomas;Padilla, Fernando;Wanner, Jutta;Xie, Wenwei;Zhang, Xiaohu US2012/252777, 2012, A1 Location in patent:Page/Page column 96
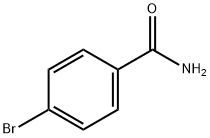
698-67-9
169 suppliers
$12.00/5g

73183-34-3
586 suppliers
$6.00/5g

179117-44-3
83 suppliers
$8.00/250mg

73183-34-3
586 suppliers
$6.00/5g

619-56-7
211 suppliers
$30.00/25 g

179117-44-3
83 suppliers
$8.00/250mg
3956-07-8
89 suppliers
$27.00/1g
25015-63-8
216 suppliers
$22.39/5G

179117-44-3
83 suppliers
$8.00/250mg

3956-07-8
89 suppliers
$27.00/1g

73183-34-3
586 suppliers
$6.00/5g

179117-44-3
83 suppliers
$8.00/250mg