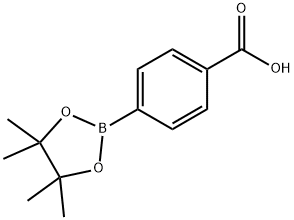
4-Carboxylphenylboronic acid pinacol ester synthesis
- Product Name:4-Carboxylphenylboronic acid pinacol ester
- CAS Number:180516-87-4
- Molecular formula:C13H17BO4
- Molecular Weight:248.08
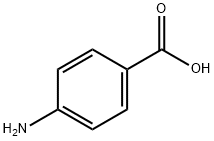
150-13-0

73183-34-3

180516-87-4
GENERAL METHODS: Tert-butyl nitrite (155 mg, 1.1 mmol) was added dropwise to a mixture containing bis(pinacolato)diboron (127 mg, 0.5 mmol), p-aminobenzoic acid (61 mg, 0.5 mmol) and eosin Y (0.01 mmol). The reaction mixture was stirred in acetonitrile (3 mL) under blue LED irradiation for 2 h at room temperature (the progress of the reaction was monitored by TLC). Upon completion of the reaction, the mixture was diluted with ethyl acetate (5 mL) and then filtered through diatomaceous earth. The filtrate was extracted with ethyl acetate (3 x 10 mL) and the combined organic phases were washed with saturated saline and dried over anhydrous Na2SO4. Concentration under reduced pressure afforded the crude product, which was purified by silica gel column chromatography using hexane-ethyl acetate (98:2) as eluent to afford pure 4-carboxyphenylboronic acid pinacol ester as a pale yellow viscous liquid (208 mg, 88% yield).IR (pure samples)2978, 2933, 2839, 2526, 2050, 1950, 1911, 1724, 1605, 1570 cm-1; 1H NMR (500 MHz, CDCl3) δ 1.33 (s, 12H), 7.82 (s, 3H), 6.89 (d, J = 8.0 Hz, 2H), 7.75 (d, J = 8.0 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 24.9 (4C), 55.2, 83.6 (2C), 113.4 (2C), 136.6 (2C), 162.3.The spectral data of the obtained compounds are in agreement with the 4-carboxyphenylboronic acid pinacol ester reported in the literature.
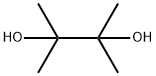
76-09-5
397 suppliers
$8.19/250mg
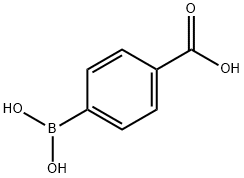
14047-29-1
471 suppliers
$10.00/5 g

180516-87-4
242 suppliers
$8.00/1g
Yield:180516-87-4 93.7%
Reaction Conditions:
in hexane at 70; for 3 h;Temperature;Time;Concentration;
Steps:
1
To a 500 mL reaction flask was added p-carboxyphenylboronic acid (50 g, 0.3 mol), pinacol (39 g, 0.33 mol), n-hexane 100 g, and the temperature was raised to 70 ° C, and the reaction was carried out for 3 hours, and concentrated under reduced pressure. Drying in vacuo gave 73 g of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoic acid as a white solid. Yield: 98%.
References:
Zhejiang Keju Bio-pharmaceutical Co., Ltd.;Yang Yong;Yang Ming;Shen Chao;Wang Dong CN109053780, 2018, A Location in patent:Paragraph 0021; 0038; 0041; 0047; 0053; 0059; 0065; 0071

150-13-0
946 suppliers
$5.00/25g

73183-34-3
587 suppliers
$6.00/5g

180516-87-4
242 suppliers
$8.00/1g
619-58-9
448 suppliers
$8.00/5g
25015-63-8
216 suppliers
$22.39/5G

180516-87-4
242 suppliers
$8.00/1g
586-76-5
504 suppliers
$10.00/1g

73183-34-3
587 suppliers
$6.00/5g

180516-87-4
242 suppliers
$8.00/1g

124-38-9
134 suppliers
$175.00/23402
195062-61-4
136 suppliers
$8.00/1g

180516-87-4
242 suppliers
$8.00/1g