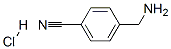
4-(Aminomethyl)benzonitrile hydrochloride synthesis
- Product Name:4-(Aminomethyl)benzonitrile hydrochloride
- CAS Number:15996-76-6
- Molecular formula:C8H9ClN2
- Molecular Weight:168.62
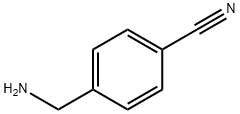
10406-25-4

15996-76-6
In a 200 mL four-neck flask equipped with a stirrer, a thermometer, a gas introduction tube, and a reflux condenser, 4-cyanobenzylamine (10.0 g) obtained in Preparation Example 3 was dissolved in ethyl acetate (90.0 g). The reactor was cooled in a water bath under stirring while hydrogen chloride gas was slowly introduced into the gas phase of the reactor. Immediately after the introduction of hydrogen chloride gas, an exothermic phenomenon was observed and a white solid precipitated. After the reaction mixture was cooled to room temperature, the white solid was separated by filtration and dried in a vacuum desiccator to give 12.6 g of 4-(aminomethyl)benzonitrile hydrochloride (99% yield as 4-cyanobenzylamine). The resulting 4-(aminomethyl)benzonitrile hydrochloride was analyzed by high performance liquid chromatography and found to contain 77 wt% of 4-cyanobenzylamine. In addition, anion chromatographic analysis showed 23 wt% of hydrogen chloride in 4-(aminomethyl)benzonitrile hydrochloride. The measured bulk density of 4-(aminomethyl)benzonitrile hydrochloride was 0.3 g/mL.

10406-25-4
171 suppliers
inquiry

15996-76-6
208 suppliers
$5.00/1g
Yield:15996-76-6 99%
Reaction Conditions:
with hydrogenchloride in ethyl acetate
Steps:
1 EXAMPLE 1
In a 200 ml four-neck flask equipped with an agitator, a thermometer, a gas conduit, and a reflux condenser, p-cyanobenzylamine (10.0 g) obtained in Preparation Example 3 was dissolved in ethyl acetate (90.0 g). While the reactor was cooled in a water bath, hydrogen chloride gas was fed into the vapor phase of the reactor while stirring. Immediately after the introduction of hydrogen chloride, heat generation was confirmed, and a white solid was precipitated. After the reaction mixture was cooled to room temperature, the white solid was separated through filtration and dried in a desiccator under vacuum, thereby yielding 12.6 g of p-cyanobenzylamine hydrochloride (yield based on p-cyanobenzylamine: 99%). Through high-performance liquid chromatographic analysis of the thus-obtained p-cyanobenzylamine hydrochloride, the p-cyanobenzylamine content in the hydrochloride was found to be 77 mass %. In addition, the hydrogen chloride content in p-cyanobenzylamine hydrochloride was found to be 23 mass % through anion chromatographic analysis. The thus-obtained p-cyanobenzylamine hydrochloride has a bulk density of 0.3 g/ml.
References:
Showa Denko K.K. US6392083, 2002, B1 Location in patent:Page column 6
17201-43-3
457 suppliers
$6.00/5g

15996-76-6
208 suppliers
$5.00/1g
15996-74-4
18 suppliers
inquiry

15996-76-6
208 suppliers
$5.00/1g
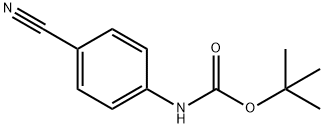
143090-18-0
59 suppliers
$22.50/1g

15996-76-6
208 suppliers
$5.00/1g
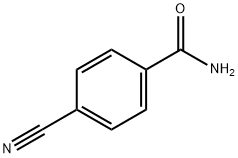
3034-34-2
111 suppliers
$55.00/250mg

15996-76-6
208 suppliers
$5.00/1g