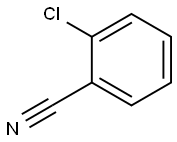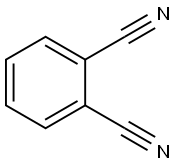
Phthalonitrile synthesis
- Product Name:Phthalonitrile
- CAS Number:91-15-6
- Molecular formula:C8H4N2
- Molecular Weight:128.13
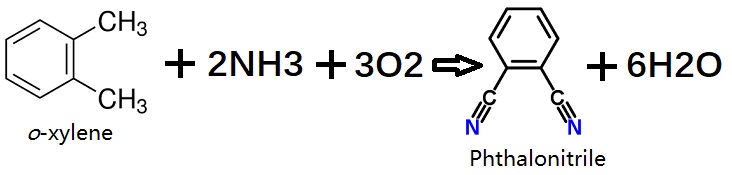
In a single-stage continuous process, o-xylene is converted to phthalonitrile by reaction with ammonia and oxygen in the gas phase in a fluidized-bed reactor. Generally, metal oxide mixtures containing vanadium, antimony, chromium, and molybdenum, with further active components such as iron, tungsten, and alkali-metal oxides, on an alumina or silica support are used as catalysts.
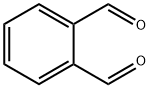
643-79-8
734 suppliers
$9.00/1g

91-15-6
270 suppliers
$13.00/50g
Yield:91-15-6 89%
Reaction Conditions:
with ammonium hydroxide;sodium persulfate;sodium iodide;iron(II) chloride in 1,2-dichloro-ethane at 20 - 50; for 16 h;
Steps:
General procedure for the synthesis of nitriles
General procedure: To a solution of aldehydes 1 (3 mmol) in 1,2-dichloroethane (10 mL) was added FeCl2(0.3 mmol), Na2S2O8 (4.5 mmol), NaI (0.15 mmol), and NH3.H2O (9 mL) at room temperature. After the mixture was stirred at 50 °C for 16 hours, it was poured into water (30 mL) and extracted with DCM (3 × 30 mL). The combined organic extracts were washed with brine and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure. The crude product was purified by column chromatography on silica gel to afford the product 2.
References:
Chen, Han;Sun, Sijia;Xi, Haoying;Hu, Kaifang;Zhang, Ning;Qu, Jingping;Zhou, Yuhan [Tetrahedron Letters,2019,vol. 60,# 21,p. 1434 - 1436] Location in patent:supporting information
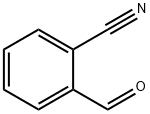
7468-67-9
249 suppliers
$13.00/5g

91-15-6
270 suppliers
$13.00/50g
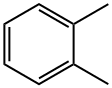
95-47-6
605 suppliers
$10.00/25g
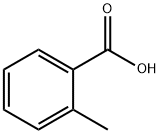
118-90-1
527 suppliers
$13.00/1g
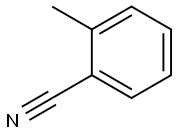
529-19-1
248 suppliers
$17.89/50g

91-15-6
270 suppliers
$13.00/50g
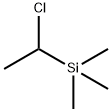
7787-87-3
44 suppliers
$181.00/5ml
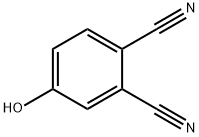
30757-50-7
129 suppliers
$60.00/100mg

91-15-6
270 suppliers
$13.00/50g