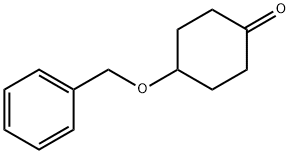
4-(Benzyloxy)cyclohexanone synthesis
- Product Name:4-(Benzyloxy)cyclohexanone
- CAS Number:2987-06-6
- Molecular formula:C13H16O2
- Molecular Weight:204.26
![1,4-DIOXA-SPIRO[4.5]DECAN-8-OL](/CAS/GIF/22428-87-1.gif)
22428-87-1

100-39-0

2987-06-6
Synthesis of 4-(benzyloxy)cyclohexanone (14-2) Procedure: a 60% sodium hydride dispersion in mineral oil (760 mg, 23 mmol, 1.2 eq.) was slowly added to a solution of anhydrous THF (50 mL) of 1,4-dioxaspiro[4.5]decan-8-ol (14-1) (3.0 g, 19 mmol, 1.0 eq.) at 0 °C. After 6 hours of reaction, benzyl bromide (2.5 mL, 21.3 mmol, 1.1 equiv) was added dropwise to the mixture. Subsequently, the reaction mixture was stirred at room temperature overnight. After the reaction was completed, 4N HCl solution (30 mL) was added and stirring was continued for 6 hours at room temperature. The reaction mixture was neutralized with 4N sodium hydroxide solution to pH ≈ 7 and then extracted with ethyl acetate (3 x 100 mL). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by an AnaLogix automated column chromatography system using a 0 to 30% ethyl acetate/heptane gradient elution to afford the target product 14-2 (3.0 g, 77% yield) as a light yellow oil.
![1,4-DIOXA-SPIRO[4.5]DECAN-8-OL](/CAS/GIF/22428-87-1.gif)
22428-87-1
203 suppliers
$6.00/1g

100-39-0
428 suppliers
$10.00/10 g

2987-06-6
100 suppliers
$11.00/1g
Yield:2987-06-6 77%
Reaction Conditions:
Stage #1:4,4-ethylenedioxycyclohexan-1-ol with sodium hydride in tetrahydrofuran;mineral oil at 0; for 6 h;
Stage #2:benzyl bromide in tetrahydrofuran;mineral oil at 20;
Stage #3: with hydrogenchloride in tetrahydrofuran;water;mineral oil at 20; for 6 h;
Steps:
5
4-(Benzyloxy)cyclohexanone (14-2): A 60% dispersion of sodium hydride in mineral oil (760 mg, 23 mmol, 1.2 equiv) was added to a solution of l ,4-Dioxaspiro[4.5]decan-8-ol (14-1) (3.0 g, 19 mmol, 1.0 equiv) in anhydrous THF (50 mL) at 0 °C. After 6 hours benzyl bromide (2.5 mL, 21.3 mmol, 1.1 equiv) was added to the mixture. The mixture was stirred at room temperature overnight. A 4N HCl solution (30 mL) was added and the reaction was stirred at room temperature for an additional 6 hours. The reaction was neutralized to pH ~7 with 4N sodium hydroxide and extracted with ethyl acetate (3 x 100 mL). The combined organic layers were dried over sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified on an AnaLogix automated column chromatography system eluting with gradient of 0 to 30% ethyl acetate in heptanes to give 14-2 (3.0 g, 77% yield) as a light yellow oil.
References:
BALANCE THERAPEUTICS, INC.;LIEN, Lyndon WO2012/151343, 2012, A1 Location in patent:Page/Page column 36
![8-(benzyloxy)-1,4-dioxaspiro[4.5]decane](/CAS2/GIF/92829-83-9.gif)
92829-83-9
28 suppliers
$308.16/1g

2987-06-6
100 suppliers
$11.00/1g
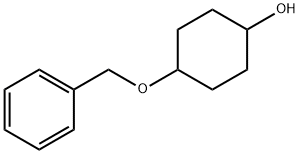
2976-80-9
36 suppliers
$50.00/25 mg

2987-06-6
100 suppliers
$11.00/1g
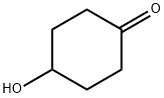
13482-22-9
203 suppliers
$8.00/250mg

100-44-7
654 suppliers
$13.50/250G

2987-06-6
100 suppliers
$11.00/1g
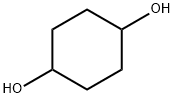
556-48-9
322 suppliers
$6.00/5g

2987-06-6
100 suppliers
$11.00/1g