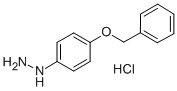
4-Benzyloxyphenylhydrazine hydrochloride synthesis
- Product Name:4-Benzyloxyphenylhydrazine hydrochloride
- CAS Number:52068-30-1
- Molecular formula:C13H15ClN2O
- Molecular Weight:250.72
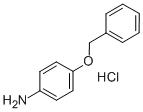
51388-20-6

52068-30-1
General procedure for the synthesis of 4-benzyloxyphenylhydrazine hydrochloride from 4-benzyloxyaniline hydrochloride: (4-(benzyloxy)phenyl)hydrazine hydrochloride (2). Note: Keep the reaction mixture and all added solutions at 0°C during this procedure. Add 4-(benzyloxy)aniline hydrochloride (3.00 g, 12.5 mmol) and water (25 mL) to the reaction flask and stir for 10 minutes at 0°C. Subsequently, a solution of NaNO2 (852 mg, 12.3 mmol) in water (6 mL) was slowly added dropwise over 15 minutes. After the dropwise addition, stirring of the reaction mixture was continued for 15 min. Then, a solution of SnCl2 (6.40 g, 33.1 mmol) in water (7.5 mL) was slowly added. After addition, the reaction mixture was stirred at 0°C for 1 hour. After completion of the reaction, the off-white precipitate was collected by filtration, washed with water and ground with ether (Et2O) to afford the target product 2 (3.01 g, 96% yield). The product characterization data were as follows: IR (ATR, pure) νmax 3232, 2906 (br), 2693, 1568, 1508, 1242, 1177 cm-1; 1H NMR (DMSO-d6, 600 MHz) δ 10.11 (bs, 3H), 7.44-7.40 (m, 2H), 7.40-7.35 (m, 2H ), 7.33-7.29 (m, 1H), 7.01-6.93 (m, 4H), 5.05 (s, 2H); 13C NMR (DMSO-d6, 150MHz) δ 153.7, 139.1, 137.3, 128.5, 128.4, 128.3, 127.9, 127.7, 127.5, 117.1, 116.9, 115.5, 116.9, 115.5, 116.9, 116.9, 115.5, 117.1 116.9, 115.5, 115.3, 69.5; HRMS (EI) m/z calculated value C13H14N2 214.1106, measured value 214.1110.

51388-20-6
263 suppliers
$9.00/10g

52068-30-1
211 suppliers
inquiry
Yield:52068-30-1 96%
Reaction Conditions:
Stage #1: 4-benzyloxyaniline hydrochloridewith hydrogenchloride;sodium nitrite in water at 0; for 0.5 h;
Stage #2: with tin(ll) chloride in water at 0; for 1 h;
Steps:
(4-(Benzyloxy)phenyl)hydrazine hydrochloride (2).; Note: The reaction mixture and all added solutions were maintained at 0 °C during this procedure. 4-Benzyloxyaniline hydrochloride (3.00 g, 12.5 mmol) was added to cone. aq. HC1 (25 mL) and stirred for 10 min at 0 °C, followed by dropwise addition of a solution of NaN02 (852 mg, 12.3 mmol) in water (6 mL) over the course of 15 min. The mixture was stirred for an additional 15 min-period and then a solution of SnCl2 (6.40 g, 33.1 mmol) in cone. aq. HC1 (7.5 mL) was added dropwise. The reaction mixture was stirred for 1 h and filtered to yield an off-white precipitate, which was washed with water and triturated with Et20, to yield 2 (3.01 g, 96%):IR (ATR, neat) 3232, 2906 (br), 2693, 1568, 1508, 1242, 1177 cm"1; 1H NMR (DMSO-d6, 600 MHz) δ 10.11 (bs, 3 H), 7.44-7.40 (m, 2 H), 7.40-7.35 (m, 2 H), 7.33-7.29 (m, 1 H), 7.01-6.93 (m, 4 H), 5.05 (s, 2 H); 13C NMR (DMSO-d6, 150 MHz) δ 153.7, 139.1, 137.3, 128.5, 128.4, 128.3, 127.9, 127.7, 127.5, 117.1, 116.9, 115.5, 115.3, 69.5; HRMS (EI) m/z calcd for Ci3Hi4N20 214.1106, found 214.1110.
References:
WO2012/78859,2012,A2 Location in patent:Page/Page column 47
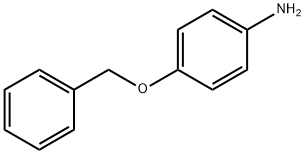
6373-46-2
164 suppliers
$6.00/1g

52068-30-1
211 suppliers
inquiry
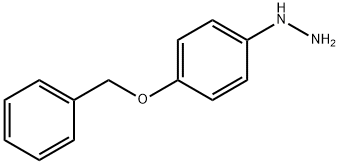
51145-58-5
34 suppliers
inquiry

52068-30-1
211 suppliers
inquiry
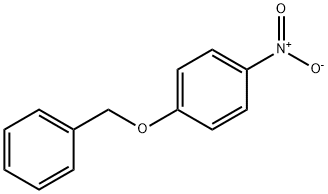
1145-76-2
109 suppliers
$26.00/5g

52068-30-1
211 suppliers
inquiry
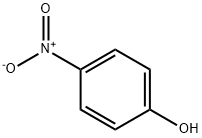
100-02-7
61 suppliers
$11.00/5G

52068-30-1
211 suppliers
inquiry