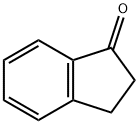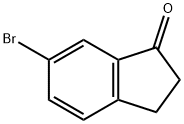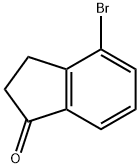
4-Bromo-1-indanone synthesis
- Product Name:4-Bromo-1-indanone
- CAS Number:15115-60-3
- Molecular formula:C9H7BrO
- Molecular Weight:211.06
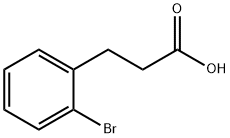
15115-58-9

15115-60-3
General procedure for the synthesis of 4-bromoinden-1-one from 3-(2-bromophenyl)propionic acid: trifluoromethanesulfonic acid (3 equiv.) was slowly added dropwise at 0 °C to an anhydrous dichloromethane (1.0 mL) solution of 3-(2-bromophenyl)propionic acid (0.5 mmol) which was placed in a 12 mL Q-tube?pressure tube (provided by QLabtech). Subsequently, the reaction mixture was warmed to room temperature. A PTFE septum was placed on top of the reaction tube and sealed using the appropriate cap and pressure adapter. The sealed reaction tube was heated in an oil bath at 80°C. The progress of the reaction was monitored by thin layer chromatography (TLC) and gas chromatography-mass spectrometry (GC/MS) until the reactants completely disappeared. After completion of the reaction, the mixture was poured into ice water and extracted three times with dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Finally, the target compound 4-bromoinden-1-one was isolated and purified from the crude product by fast column chromatography.
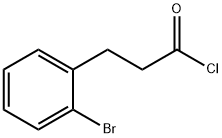
90725-40-9
5 suppliers
inquiry

15115-60-3
336 suppliers
$5.00/1g
Yield:15115-60-3 435 g
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 23 - 27; for 3 h;
Steps:
4-Bromo-2,3-dihydro-1H-inden-1-one
3-(2-Bromophenyl)propanoic acid (1) (550 g, 2.4 mol, 1 equiv) was dissolved in 1,2-dichloroethane (5.5 L). Thionyl chloride (437.8 mL, 6 mol, 2.5 equiv) was added to the solution and the mixture was refluxed for 24 hours. The reaction was cooled to room temperature and concentrated under reduced pressure. The residue was dissolved in methylene chloride (1 L) and added dropwise to a mechanically stirred suspension of anhydrous aluminum chloride (526.9 g, 3.96 mol, 1.65 equiv) in dichloromethane (1 L) while keeping the reaction temperature below 27° C. The reaction was stirred at room temperature for three hours before being quenched into a five gallon bucket which was half-full of ice. The resulting mixture was extracted with dichloromethane (3×3 L). The combined organic layers were washed sequentially with saturated brine (2 L) and saturated sodium bicarbonate (2 L). The organic layer was dried over sodium sulfate, and concentrated under reduced pressure. The resulting solid was dried overnight in a vacuum oven at 30° C. to give compound 2 (435 g, 86% yield) as an off-white solid.
References:
US9249239,2016,B2 Location in patent:Page/Page column 51

15115-58-9
206 suppliers
$14.00/1g

15115-60-3
336 suppliers
$5.00/1g
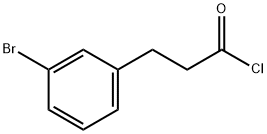
335159-82-5
3 suppliers
inquiry

15115-60-3
336 suppliers
$5.00/1g

79-37-8
489 suppliers
$17.67/10gm:

15115-58-9
206 suppliers
$14.00/1g

15115-60-3
336 suppliers
$5.00/1g