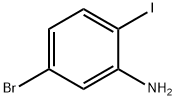
5-BROMO-2-IODOANILINE synthesis
- Product Name:5-BROMO-2-IODOANILINE
- CAS Number:64085-52-5
- Molecular formula:C6H5BrIN
- Molecular Weight:297.92
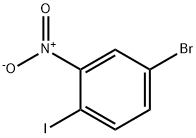
112671-42-8

64085-52-5
The general procedure for the synthesis of 5-bromo-2-iodoaniline from 4-bromo-1-iodo-2-nitrobenzene was as follows: 4-bromo-1-iodo-2-nitrobenzene (8.38 g, 109.6 mmol) was dissolved in a mixed solvent of acetic acid (AcOH, 45 mL) and ethanol (EtOH, 45 mL) at room temperature, followed by the addition of powdered iron (Fe, 6.12 g 109.6 mmol). The reaction mixture was heated to 100 °C and stirred for 1.3 h, after which it was cooled to room temperature. The reaction mixture was diluted with ether (Et2O, 90 mL) and washed with saturated aqueous sodium bicarbonate (NaHCO3) solution (200 mL). The organic layer was separated and the aqueous layer was further extracted with ether (3 x 60 mL). All organic layers were combined, washed with brine (300 mL), dried over anhydrous sodium sulfate (Na2SO4) and subsequently concentrated under reduced pressure. The residue was purified by fast column chromatography (silica gel as stationary phase, hexane/ethyl acetate=19:1 as eluent) to afford the target product 5-bromo-2-iodoaniline (6.89 g, 91% yield) as a light yellow solid.

112671-42-8
156 suppliers
$9.00/1g

64085-52-5
128 suppliers
$5.00/250mg
Yield: 93.6%
Reaction Conditions:
with iron;ammonium chloride in tetrahydrofuran;ethanol;water at 70; for 3 h;
Steps:
1.1
A mixture of 4-bromo-1-iodo-2-nitro-benzene (2 g, 6.10 mmol, 1 eq), NH4Cl (1.63 g, 30.50 mmol, 5 eq), Fe (1.70 g, 30.50 mmol, 5 eq) in EtOH (8 mL), THF (8 mL) and H2O (8 mL) was degassed and purged with N2 for 3 times. The mixture was stirred under N2 atmosphere at 70 °C for 3 h. TLC (PE/EtOAc = 5/1, Rf = 0.45) indicated starting material was consumed completely and two new spots formed. The mixture was concentrated and water (80 mL) was added. The mixture was extracted with EtOAc (50 mL x 3) and the combined organic layers were washed with brine (50 mL), dried over Na2SO4, filtered and concentrated to yield a residue which was purified by flash silica gel chromatography (From PE/EtOAc = 1/0 to 5/1, Rf = 0.45) to yield 5-bromo-2- iodo-aniline (1.7 g, 5.71 mmol, 93.6% yield, 100.0% purity) as a light yellow solid.1H NMR (400 MHz, DMSO-d6) d ppm 7.43 (d, J = 8.2 Hz, 1H), 6.89 (d, J = 2.0 Hz, 1H), 6.45 (dd, J = 2.0, 8.2 Hz, 1H), 5.47 (s, 2H); ES-LCMS m/z 298.0, 300.0 [M+H]+.
References:
IKENA ONCOLOGY, INC.;CASTRO, Alfredo C. WO2020/243423, 2020, A1 Location in patent:Paragraph 00465; 00790
875-51-4
305 suppliers
$5.00/1g

64085-52-5
128 suppliers
$5.00/250mg
881-50-5
66 suppliers
$8.00/100mg

64085-52-5
128 suppliers
$5.00/250mg
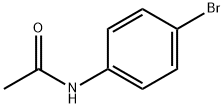
103-88-8
273 suppliers
$6.00/5g

64085-52-5
128 suppliers
$5.00/250mg
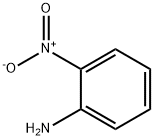
88-74-4
58 suppliers
$10.00/1g

64085-52-5
128 suppliers
$5.00/250mg