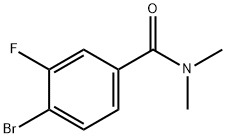
4-BROMO-3-FLUORO-N,N-DIMETHYLBENZAMIDE synthesis
- Product Name:4-BROMO-3-FLUORO-N,N-DIMETHYLBENZAMIDE
- CAS Number:893420-59-2
- Molecular formula:C9H9BrFNO
- Molecular Weight:246.08
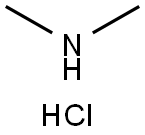
506-59-2
556 suppliers
$5.00/5G
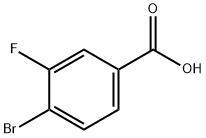
153556-42-4
262 suppliers
$10.00/1g

893420-59-2
14 suppliers
inquiry
Yield:893420-59-2 99%
Reaction Conditions:
Stage #1: 4-bromo-3-fluorobenzoic acidwith thionyl chloride;N,N-dimethyl-formamide at 10 - 80; for 2 h;
Stage #2: N,N-dimethylammonium chloridewith triethylamine in dichloromethane at 0 - 15; for 2 h;
Steps:
67.1 Step 7: Preparation of 4-bromo-3-fluoro-N,N-dimethylbenzamide
To a suspension of 4-bromo-3-fluorobenzoic acid (20.0 g, 91.3 mmol) in SOCh (100 mL) was added DMF (0.5 mL) at 10-15 °C. Then the reaction mixture was heated at 80 °C for 2 h. The mixture turned into yellow solution from suspension. The reaction mixture was concentrated and the residue was diluted with anhydrous toluene (50 mL) and concentrated in turn for 3 times to remove most of SOCh. Then the residue was dissolved in anhydrous DCM (50 mL) and added dropwise to a mixture of dimethylamine HC1 salt (14.9 g, 183 mmol) and EbN (37.0 g, 365 mmol) in anhydrous DCM (150 mL) at 0 °C. After the completion of the addition, the reaction mixture was stirred at 10-15 °C for 2 h. A lot of precipitate was formed after stirring. To the reaction mixture was added water (100 mL), then extracted with DCM (100 mL x3). The combined organic layer was washed with 1N aqueous HC1 (100 mL), 1N aqueous NaOH (100 mL), brine (100 mL), dried over anhydrous Na2S04 and concentrated to give 4-bromo-3-fluoro-N,N-dimethylbenzamide (22.3 g, yield: 99%) as a yellow solid. (1008) NMR (400 MHz, CDCb) d 2.99 (3H, s), 3.10 (3H, s), 7.10 (1H, dd, J= 8.2, 1.3 Hz), 7.20 (1H, dd, J= 8.6, 1.9 Hz), 7.60 (1H, dd, J= 8.2, 6.9 Hz).
References:
WO2019/126730,2019,A1 Location in patent:Paragraph 0386

124-40-3
545 suppliers
$18.00/100ml

893420-59-2
14 suppliers
inquiry

124-40-3
545 suppliers
$18.00/100ml

153556-42-4
262 suppliers
$10.00/1g

893420-59-2
14 suppliers
inquiry