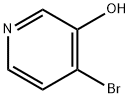
4-BROMO-3-HYDROXYPYRIDINE synthesis
- Product Name:4-BROMO-3-HYDROXYPYRIDINE
- CAS Number:161417-28-3
- Molecular formula:C5H4BrNO
- Molecular Weight:174
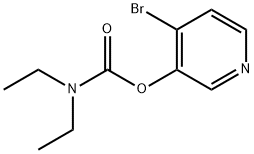
98976-81-9

161417-28-3
The general procedure for the synthesis of 4-bromo-3-hydroxypyridine from the compound (CAS: 98976-81-9) is as follows: to a solution of 4-bromo-3-pyridinyl diethylcarbamate (1.24 g, 4.50 mmol) in methanol (10 mL) was added methanolic solution of sodium methanolate (2.04 g, 9.40 mmol) at 20°C and the resulting mixture was refluxed for 1.5 hours. Upon completion of the reaction, methanol was removed by distillation under reduced pressure, ethyl acetate (15 mL) and water (1 mL) were added, and then the pH was adjusted to 9 with 20% aqueous sulfuric acid. the organic layer was separated, and the residue was washed with ethyl acetate (3 x 5 mL). The organic layers were combined, dried and concentrated with anhydrous sodium sulfate. The crude product was purified by rapid chromatography on silica gel, eluting sequentially with hexane/ethyl acetate (1:1 and 1:2) to give 691 mg (89% yield) of 4-bromo-3-hydroxypyridine.TLC Rf value was 0.38 (1:2 hexane/ethyl acetate). Mass spectra (DCI/NH3) m/e 174 (with 79 Br) and 176 (M+H)+ (with 81 Br).1H NMR (CDCl3, 300 MHz) δ: 8.43 (d, J=1.5 Hz, 1H, ArH), 8.02 (d, J=7.2 Hz, 1H, ArH), 7.54 (dd, J=7.2,1.5 Hz. 1H, ArH).

98976-81-9
1 suppliers
inquiry

161417-28-3
158 suppliers
$15.00/100mg
Yield:161417-28-3 89%
Reaction Conditions:
with sodium methylate in methanol
Steps:
20.c 20c.
20c. 4-bromo-3-pyridinol To a solution of 4bromo-3-pyridyl diethylcarbamate (1.24 g, 4.50 mmol) in methanol (10 mL) was added sodium methoxide in methanol (2.04 g, 9.40 mmol), and the resultant mixture was refluxed for 1.5 hours. After removal of MeOH, EtOAc (15 mL) and water (1 mL) were added, the pH was then adjusted to 9 using 20% H2 SO4. The organic layer was decanted, and the residue washed with EtOAc (3*5 mL). The combined organic layers were dried (Na2 SO4) and concentrated. The crude product was purified by flash chromatography on silica gel eluding with hexane/EtOAc (1:1 and 1:2) to provide 691 mg (89% yield) of the title compound. TLC Rf 0.38 (1:2 hexane/EtOAc). MS (DCI/NH3) m/e 174 with 79 Br and 176 (M+H)+ with 81 Br. 1 H NMR (CDCl3, 300 MHz) δ: 8.43 (d, J =1.5 Hz, 1H, ArH), 8.02 (d, J=7.2 Hz, 1H, ArH), 7.54 (dd, J=7.2, 1.5 Hz, 1H, ArH).
References:
Abbott Laboratories US5914328, 1999, A
10182-48-6
79 suppliers
$106.00/100mg

161417-28-3
158 suppliers
$15.00/100mg