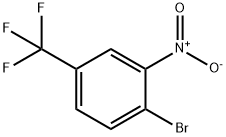
4-Bromo-3-nitrobenzotrifluoride synthesis
- Product Name:4-Bromo-3-nitrobenzotrifluoride
- CAS Number:349-03-1
- Molecular formula:C7H3BrF3NO2
- Molecular Weight:270
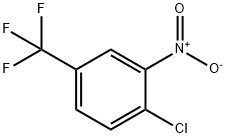
121-17-5

349-03-1
Example 10: Synthesis of 4-bromo-3-nitrobenzotrifluoride from 4-chloro-3-nitrobenzotrifluoride (g); [0086] 4-chloro-3-nitrobenzotrifluoride (1 eq.) was synthesized from 4-chloro-3-nitrobenzotrifluoride (1 eq.) with a source of bromide (cuprous bromide, cupric bromide, lithium bromide, or magnesium bromide, 1 eq.) or a mixture of cuprous bromide (1 eq.) with lithium bromide (1 eq.) under the presence of benzyl cyanide, diethylene glycol dimethyl ether ( diethylene glycol dimethyl ether), acetic acid or N-methylpyrrolidone (1 mL/mmol) as solvent, and heated the reaction at 160 °C for 6 h to obtain the target product 4-bromo-3-nitrobenzotrifluoride. Table 11 shows that good selectivity was obtained using different reaction conditions, with the mixture of cuprous bromide and lithium bromide being particularly effective. Comparative experiments showed that the reaction was less effective under solvent-free conditions.

121-17-5
32 suppliers
$19.00/250g

349-03-1
216 suppliers
$8.00/5g
Yield:349-03-1 38%
Reaction Conditions:
with copper(I) bromide;lithium bromide in tetrahydrofuran at 160; for 6 h;Product distribution / selectivity;
Steps:
11
The above reaction was repeated but using acetic acid, acetonitrile or tetrahydrofuran instead of benzonitrile at various concentrations (0.02, 0.04, 0.1 and 1 ml/mmol). Table 14 shows the results, from which it may be seen that the optimal concentration depends upon the solvent, and that the best results were obtained at high concentration.
References:
Bayer CropScience S.A. US6635780, 2003, B1 Location in patent:Page/Page column 12
320-94-5
235 suppliers
$9.00/1g

349-03-1
216 suppliers
$8.00/5g
402-43-7
445 suppliers
$6.00/10g

349-03-1
216 suppliers
$8.00/5g