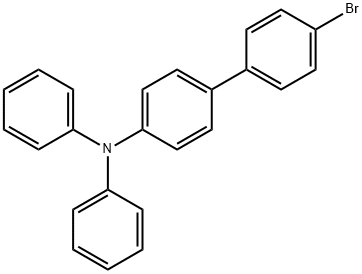
4-BROMO-4'-(DIPHENYLAMINO)BIPHENYL synthesis
- Product Name:4-BROMO-4'-(DIPHENYLAMINO)BIPHENYL
- CAS Number:202831-65-0
- Molecular formula:C24H18BrN
- Molecular Weight:400.31
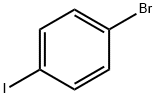
589-87-7
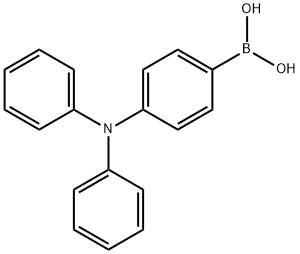
201802-67-7

202831-65-0
Example 1.13.1 Synthesis of 4'-bromo-N,N-diphenyl-[1,1'-biphenyl]-4-amine (35): (4-(diphenylamino)phenyl)boronic acid (1.5 g, 5.19 mmol), 4-iodo-1-bromobenzene (1.33 g, 4.71 mmol), Na2CO3 (1.78 g, 16.8 mmol) and Pd(PPh3 )4 (0.163 g, 0.141 mmol) were dissolved in THF/H2O (28 mL/17 mL) mixed solvent. The reaction mixture was degassed and heated to reflux under argon protection overnight. After completion of the reaction, it was cooled to room temperature and the mixture was poured into dichloromethane (150 mL) and washed sequentially with water (2 x 150 mL) and brine (100 mL). The organic phase was dried with Na2SO4, filtered and purified by fast column chromatography (silica gel, hexane/ethyl acetate 50:1), and finally recrystallized in a solvent mixture of dichloromethane/methanol to give a white solid product (compound 35) (1.64 g, 87% yield).
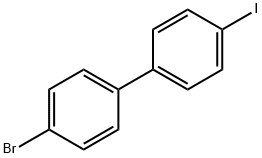
105946-82-5
236 suppliers
$10.00/10g
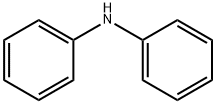
122-39-4
357 suppliers
$14.00/5g

202831-65-0
152 suppliers
$19.00/250mg
Yield: 78%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;sodium t-butanolate in tolueneInert atmosphere;Reflux;Sonication;
Steps:
1 Example 1: Preparation of Intermediate A-1
Compound I-1 (12.5 g, 73.8 mmol), 4-bromo-4'-iodide (26.5 g, 73.8 mmol) were sequentially added to a round bottom flask.t-BuONa (10.7 g, 111 mmol), Pd(dppf)Cl2.CH2Cl2 (1.2 g, 1.47 mmol), and ultrasonically deoxygenated toluene (1.5 L) were refluxed under nitrogen atmosphere overnight. After cooling the reaction solution, ethyl acetate was used. Ester and water treatment, and the resulting organic layer was dried over MgSO 4Evaporate the solvent under reduced pressureThe crude product of compound II-1 was obtained.With silicone as the stationary phase,Dichloromethane/hexane as eluent,The crude product is subjected to column chromatography.Compound II-1 (23.0 g, 78%) was obtained.
References:
Changchun Hai Purunsi Technology Co., Ltd.;Liu Xiqing;Sun Jing CN107827873, 2018, A Location in patent:Paragraph 0048; 0049; 0050

589-87-7
540 suppliers
$10.00/1g

201802-67-7
285 suppliers
$6.00/1g

202831-65-0
152 suppliers
$19.00/250mg
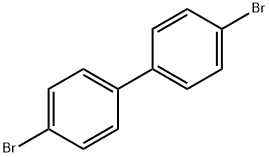
92-86-4
394 suppliers
$15.00/25g

122-39-4
357 suppliers
$14.00/5g

202831-65-0
152 suppliers
$19.00/250mg
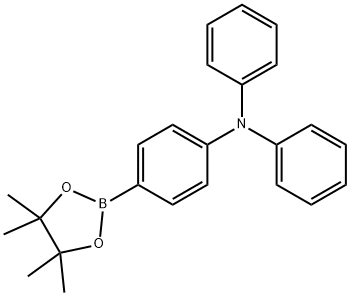
267221-88-5
102 suppliers
$10.00/250mg

589-87-7
540 suppliers
$10.00/1g

202831-65-0
152 suppliers
$19.00/250mg
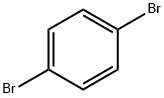
106-37-6
479 suppliers
$9.00/5g

201802-67-7
285 suppliers
$6.00/1g

202831-65-0
152 suppliers
$19.00/250mg