
4-BROMODIBENZOFURAN synthesis
- Product Name:4-BROMODIBENZOFURAN
- CAS Number:89827-45-2
- Molecular formula:C12H7BrO
- Molecular Weight:247.09
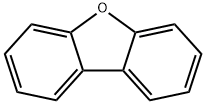
132-64-9

89827-45-2
Under nitrogen protection, 33.6 mg (200 mmol) of dibenzo[b,d]furan was dissolved in 700 mL of tetrahydrofuran (THF). After cooling the reaction system to -70 °C or lower, 350 mL of n-butyllithium (1.6 mol/L hexane solution) was slowly added. After addition, the reaction mixture was stirred by maintaining the temperature at -70°C or lower for 1 hour. Subsequently, the reaction system was warmed to 0°C and stirring was continued for 1 hour. The reaction system was again cooled to -70 °C or lower and 45 g (300 mL) of 1,2-dibromoethane was added dropwise. After the dropwise addition was completed, the reaction mixture was stirred at -70 °C or lower for 12 hours. Upon completion of the reaction, water was added to the reaction mixture and the product was extracted with ethyl acetate. The organic layers were combined, washed with water and concentrated under reduced pressure to remove the solvent. The resulting residue was recrystallized with hexane to give 37.1 g of Intermediate 1 in 75% yield. The structure of the product was confirmed by nuclear magnetic resonance (NMR) and mass spectrometry (MS) analysis.
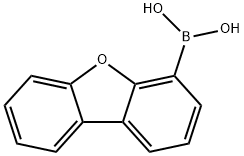
100124-06-9
401 suppliers
$6.00/1g

89827-45-2
205 suppliers
$7.00/1g
Yield:89827-45-2 89%
Reaction Conditions:
with tetrabutylammomium bromide;copper(ll) bromide in water at 100;Sealed tube;
Steps:
1. Preparation of aryl bromides
General procedure: To a stirred solution of arylboronic acids 1 (1 mmol), CuBr2 (335 mg, 1.5 mmol), TBAB (33 mg, 0.1 mmol) water (5mL) in a sealed tube for 5-12 h at 100 °C. After completion of the reaction as indicated by TLC, the mixture was extracted with EtOAc (2×5 mL). The organic layer was dried by anhydrous Na2SO4, concentrated in vacuo, and the residue was isolated by simply column chromatography (PE/EA 40:1-20:1) to afford products, respectively.
References:
Tang, Yan-Ling;Xia, Xian-Song;Gao, Jin-Chun;Li, Min-Xin;Mao, Ze-Wei [Tetrahedron Letters,2021,vol. 64,art. no. 152738] Location in patent:supporting information

132-64-9
364 suppliers
$5.00/10g

89827-45-2
205 suppliers
$7.00/1g
![[1,1'-Biphenyl]-2-ol, 3-bromo-](/CAS/20210305/GIF/23197-48-0.gif)
23197-48-0
6 suppliers
inquiry

89827-45-2
205 suppliers
$7.00/1g
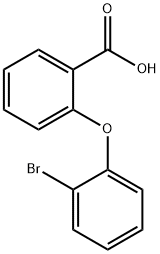
917477-33-9
4 suppliers
inquiry

89827-45-2
205 suppliers
$7.00/1g
