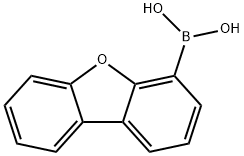
Dibenzofuran-4-boronic acid synthesis
- Product Name:Dibenzofuran-4-boronic acid
- CAS Number:100124-06-9
- Molecular formula:C12H9BO3
- Molecular Weight:212.01

89827-45-2
204 suppliers
$7.00/1g

5419-55-6
388 suppliers
$12.00/5g

100124-06-9
403 suppliers
$6.00/1g
Yield:100124-06-9 94%
Reaction Conditions:
Stage #1: 4-bromodibenzofuranwith n-butyllithium in tetrahydrofuran at -78; for 1.5 h;Inert atmosphere;
Stage #2: Triisopropyl borate in tetrahydrofuran at 20; for 8 h;
Steps:
4.1 (5-(4-([2,2':6',2"-teridopyridine]-4'-yl)phenyl)-5H-benzofuro[3,2-c]indole)Preparation
(1) 4-bromodibenzofuran (7.4 g, 30 mmol, 1 eq) in a 50 ml three-necked flask,Add dry tetrahydrofuran solution, cool to -78 ° C, replace N2, three times,To ensure the oxygen-free environment of the system, add n-butyl lithium (2.5M, 13.2ml,After 33 mmol, 1.1 eq), the reaction was kept at -78 ° C for 1.5 h, and triisopropyl borate (ρ = 0.815 g/ml, 7.6 ml, 33 mmol, 1.1 eq) was added dropwise. After the addition was completed, the temperature was raised to room temperature for 8 h. After the dropwise addition of dilute hydrochloric acid, the reaction was quenched, distilled under reduced pressure to give tetrahydrofuran, dissolved in dichloromethane, washed with anhydrous magnesium sulfate, dried, filtered, and the filtrate was recrystallized.White solid product4-boronic acid dibenzofuran6 g (yield 94%).
References:
CN109897029,2019,A Location in patent:Paragraph 0063-0066

89827-45-2
204 suppliers
$7.00/1g

121-43-7
381 suppliers
$14.00/25mL

100124-06-9
403 suppliers
$6.00/1g
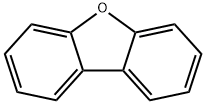
132-64-9
367 suppliers
$5.00/10g

121-43-7
381 suppliers
$14.00/25mL

7732-18-5
508 suppliers
$13.50/100ML

100124-06-9
403 suppliers
$6.00/1g

132-64-9
367 suppliers
$5.00/10g
28049-80-1
0 suppliers
inquiry

7732-18-5
508 suppliers
$13.50/100ML

100124-06-9
403 suppliers
$6.00/1g

132-64-9
367 suppliers
$5.00/10g
6562-41-0
12 suppliers
inquiry

7732-18-5
508 suppliers
$13.50/100ML

100124-06-9
403 suppliers
$6.00/1g