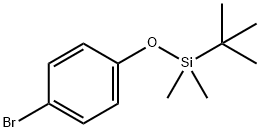
(4-BROMOPHENOXY)-TERT-BUTYLDIMETHYLSILANE synthesis
- Product Name:(4-BROMOPHENOXY)-TERT-BUTYLDIMETHYLSILANE
- CAS Number:67963-68-2
- Molecular formula:C12H19BrOSi
- Molecular Weight:287.27
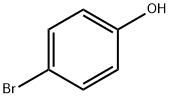
106-41-2

18162-48-6

67963-68-2
Step 1a: 4-Bromophenol (10.0 g, 57.8 mmol) and tert-butyldimethylchlorosilane (TBSCl, 11.3 g, 75.14 mmol) were sequentially added to dry DMC (100 mL). Triethylamine (Et3N, 16.7 g, 115.6 mmol) was slowly added dropwise at room temperature, and the reaction mixture was continued to be stirred for 2 h after the dropwise addition. After completion of the reaction, the solvent was removed by distillation under reduced pressure. Petroleum ether (200 mL) was added to the residue, transferred to a partition funnel, and the organic layer was washed sequentially with water and saturated saline. The organic layer was dried with anhydrous sodium sulfate (Na2SO4), filtered and purified by a short silica gel column, and finally the solvent was evaporated under reduced pressure to give the target product (4-bromophenoxy)tert-butyldimethylsilane (compound 0101, 16.6 g, 100% yield) as a colorless oil.1H NMR (CDCl3) δ: 0.18 (s, 6H), 0.98 (s, 9H), and 2.71 (t, J = 6 Hz, 2H), 6.70-6.73 (m, 2H), 7.30-7.33 (m, 2H).
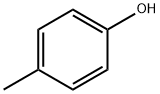
106-44-5
502 suppliers
$14.00/5g

18162-48-6
677 suppliers
$9.00/5g

67963-68-2
136 suppliers
$17.00/1g
Yield: 56%
Reaction Conditions:
Stage #1:p-cresol;tert-butyldimethylsilyl chloride with 1H-imidazole in DMF (N,N-dimethyl-formamide) at 40; for 2 h;
Stage #2: with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane for 0.5 h;Sun lamp;Heating / reflux;
Steps:
Phenolic stilbene 57Z was obtained via a route employing a TBS-protecting group (Scheme 3). TBS-protected benzylic bromide 58 (Giral, L.; Montginoul, C.; Schue, R. S. et F. Bull. Soc. Chim. Belg. 1990, 99, 147-166) was prepared in 2 steps by TBS protection (Stern, A. J.; Swenton, J. S. J. Org. Chem. 1989, 54, 2953-2958) and NBS bromination. Phosphonium bromide 59 (Giral, L.; Montginoul, C.; Schue, R. S. et F. Bull. Soc. Chim. Belg. 1990, 99, 147-166) was prepared by two different methods: In one method, a neat mixture of benzylic bromide 58 and PPh3 was heated to melt with a heat gun for a few minutes to complete the reaction. (Stern, A. J.; Swenton, J. S. J. Org. Chem. 1989, 54, 2953-2958.) The second method was heating up the reaction mixture of benzylic bromide 58 and PPh3 at 8090° C. in THF over 6 hours. [00054] The Wittig reaction of 4-biphenylcarboxaldehyde with the phosphonium ylide generated from the phosphonium bromide 59 by the deprotonation using KHMDS provided the TBS protected stilbene 60Z in 44% yield. Desilylation with tetra-n-butylammonium fluoride (TBAF) (Corey, E. J.; Venkateswarlu, A. J. Am. Chem. Soc. 1972, 94, 6190-6191) in THF afforded phenol 57Z in quantitative yield. Rf0.24 (Hexanes/CH2Cl2 1/1); mp 97-103° C.; IR (KBr) 3530, 1608, 1508, 1485, 1255, 1171 cm-1; 1H NMR (300 MHz, CDCl3) δ7.61 (d, 2 H, J=7.3 Hz), 7.51-7.30 (m, 7 H), 7.21 (d, 2 H, J=8.5 Hz), 6.72 (d, 2 H, J=8.5 Hz), 6.55 (AB q, 2 H, J=12.6 Hz); 13C NMR (75 MHz, CDCl3) δ154.55, 140.64, 139.52, 136.46, 130.35, 129.81, 129.24, 128.74, 128.41, 127.23, 126.84, 126.81, 115.11; HRMS calcd for C20H16O 272.1201, found 272.1201.
References:
University of Pittsburgh US6706839, 2004, B1 Location in patent:Page column 15

106-41-2
498 suppliers
$13.00/5g

18162-48-6
677 suppliers
$9.00/5g

67963-68-2
136 suppliers
$17.00/1g
18052-27-2
1 suppliers
inquiry

67963-68-2
136 suppliers
$17.00/1g