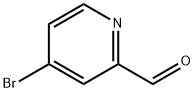
4-BROMOPYRIDINE-2-CARBALDEHYDE synthesis
- Product Name:4-BROMOPYRIDINE-2-CARBALDEHYDE
- CAS Number:131747-63-2
- Molecular formula:C6H4BrNO
- Molecular Weight:186.01
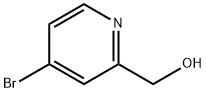
131747-45-0

131747-63-2
General procedure for the synthesis of 4-bromopyridine-2-carboxaldehyde from 2-hydroxymethyl-4-bromopyridine: 3L of dichloromethane (DCM) was added to a 5L three-necked flask under nitrogen protection, and cooled to -60 °C. During the cooling process, 240 g of oxalyl chloride was added slowly and dropwise. Maintaining the reaction temperature at -60 °C, 295.6 g of dimethyl sulfoxide (DMSO) was added dropwise to the reaction system and held for 30 min after the dropwise addition. Subsequently, 237.5 g of 2-hydroxymethyl-4-bromopyridine (Cpd 3) was slowly added dropwise at -60 °C and the reaction was continued at -65 °C for 1 hour. At this temperature, 3.5 equivalents of triethylamine (TEA) was added, and the reaction solution was allowed to stand before the progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the target product 4-bromopyridine-2-carboxaldehyde (Compound 4) was purified by column chromatography to give 177.2 g in 75% yield.

131747-45-0
186 suppliers
$13.00/50mg

131747-63-2
170 suppliers
$25.00/250mg
Yield:131747-63-2 100%
Reaction Conditions:
with manganese(IV) oxide in chloroform for 0.75 h;Reflux;
Steps:
29.i 4-Bromopicolinaldehyde
Example 29i 4-Bromopicolinaldehyde Manganese(IV) oxide (22.19 g, 255.29 mmol) was added to a solution of (4-bromopyridin-2-yl)methanol (4.00 g, 21.27 mmol) in chloroform (80 mL) and the reaction mixture was stirred under reflux for 45 min. After the mixture had cooled to room temperature the solids were removed by filtration through a pad of Celite. The solvent was removed in vacuo and the residue (3.96 g, quant.) was used without further purification in the next step. 1H NMR (500 MHz, DMSO-d6) δ ppm 9.97 (s, 1H) 8.80 (d, 1H) 7.98 (d, 1H) 7.88 (dd, 1 H); MS (APCI+): m/z 186, 188 [M+H]+.
References:
ASTRAZENECA AB US2010/125082, 2010, A1 Location in patent:Page/Page column 24
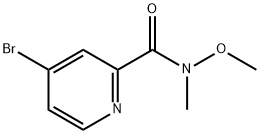
1005342-94-8
12 suppliers
inquiry

131747-63-2
170 suppliers
$25.00/250mg
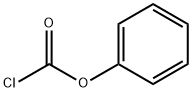
1885-14-9
345 suppliers
$10.00/1g

131747-63-2
170 suppliers
$25.00/250mg
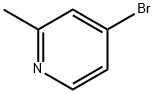
22282-99-1
405 suppliers
$6.00/5g

131747-63-2
170 suppliers
$25.00/250mg
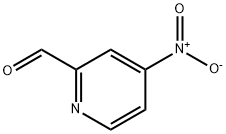
108338-19-8
52 suppliers
$112.00/100mg

131747-63-2
170 suppliers
$25.00/250mg