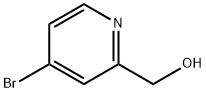
4-Bromo-2-pyridinemethanol synthesis
- Product Name:4-Bromo-2-pyridinemethanol
- CAS Number:131747-45-0
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02
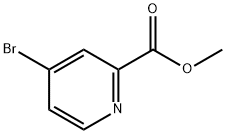
29681-42-3

131747-45-0
Step 1: Methyl 4-bromopyridine-2-carboxylate (990 mg, 4.58 mmol) and ethanol (250 mL) were added to a 250 mL reaction flask. Sodium borohydride (380 mg, 10 mmol) was added slowly in batches under stirring conditions. The reaction mixture was stirred at room temperature and under nitrogen protection for 18 hours. After completion of the reaction, 5 mL of acetone was added to the system and stirring was continued for 15 minutes. The reaction solution was filtered, and the filtrate was concentrated and extracted by adding ethyl acetate and water to separate the organic and aqueous phases. The organic phase was dried and concentrated to give 2-hydroxymethyl-4-bromopyridine (yellow liquid, 760 mg, 88% yield), and the crude product was used directly in the next reaction. (MS data: [M + 1] 187.9)

29681-42-3
193 suppliers
$5.00/250mg

131747-45-0
186 suppliers
$13.00/50mg
Yield:131747-45-0 94%
Reaction Conditions:
with sodium tetrahydroborate;ethanol at 20; for 18 h;Inert atmosphere;
Steps:
17
Example 17, Compound 17; (E)-(1 S,14R,15R,18S,21 S)-21 -(3-Hydroxy-benzyl)-18-isopropyl-14-methoxy-15-methyl-3-oxa-6,17,20,23,27-pentaaza-tricyclo[21.3.1.1 *5,9*]octacosa- 5 7,9(28),10-tetraene-2,16,19,22-tetraone; Com ound 17a: (4-Bromo-pyridin-2-yl)-methanol.; Sodium borohydride (763 mg, 20.17 mmol) was added portionwise to a solution of 4- bromo-pyridine-2-carboxylic acid methyl ester (1 .98 g, 9.166 mmol) in ethanol (50 mL) under nitrogen and the reaction mixture was stirred at room temperature for 18 hours. The reaction was quenched by the addition of acetone (10 mL) and the reaction was stirred for 15 minutes. The solvent was evaporated and the residue partitioned between water and ethyl acetate. The aqueous layer was extracted with ethyl acetate and the combined organics were collected, dried over anhydrous sodium sulfate and the solvent evaporated to afford the title compound (1 .61 g, 94%) as a yellow oil. H NMR (300 MHz, CDCI3) 3.4-3.5 {br s, 1 H), 4.80 (s, 2H), 7.40 (dd, J = 5.4, 1 .8 Hz, 1 H), 7.50 (br m, 1 H), 8.39 (d, J = 5.4 Hz, 1 H). LCMS (m/z) 188/200 [M+H], Tr = 1 .55 min.
References:
GILEAD SCIENCES, INC.;SELCIA LIMITED;APPLEBY, Todd;FLIRI, Hans, G.;KEATS, Andrew, J.;LAZARIDES, Linos;MACKMAN, Richard, L.;PETTIT, Simon, N.;POULLENNEC, Karine, G.;SANVOISIN, Jonathan;STEADMAN, Victoria, A.;WATT, Gregory, M. WO2012/78915, 2012, A1 Location in patent:Page/Page column 95-96
100367-74-6
27 suppliers
inquiry

131747-45-0
186 suppliers
$13.00/50mg
30766-03-1
271 suppliers
$7.00/1g

131747-45-0
186 suppliers
$13.00/50mg
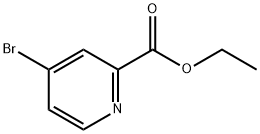
62150-47-4
101 suppliers
$32.00/250mg

131747-45-0
186 suppliers
$13.00/50mg

100367-74-6
27 suppliers
inquiry
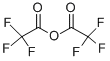
407-25-0
470 suppliers
$9.00/1g

131747-45-0
186 suppliers
$13.00/50mg