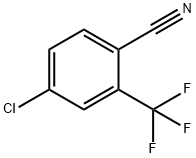
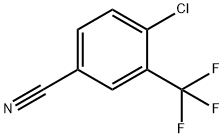
4-Chloro-2-(trifluoromethyl)benzonitrile synthesis
- Product Name:4-Chloro-2-(trifluoromethyl)benzonitrile
- CAS Number:320-41-2
- Molecular formula:C8H3ClF3N
- Molecular Weight:205.56
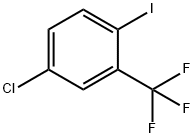
23399-77-1
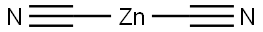
557-21-1

320-41-2
General procedure for the synthesis of 2-trifluoromethyl-4-chlorobenzonitrile from 2-iodo-5-chlorobenzotrifluoride and zinc cyanide: 4-chloro-1-iodo-2-trifluoromethylbenzene (1500 mg, 4.89 mmol), zinc cyanide (345 mg, 2.94 mmol) and tetrakis(triphenylphosphine)palladium(0) (564 mg, 0.488 mmol), then N,N-dimethylformamide (40 mL) was added. The reaction mixture was heated at 80 °C for overnight reaction. After the reaction was completed, it was cooled to room temperature, the reaction solution was diluted with toluene, washed sequentially with 2N ammonium hydroxide solution (3 times) and saturated aqueous sodium chloride solution, and the organic phase was filtered after drying and concentrated to obtain the crude product. The crude product was purified by silica gel column chromatography with hexane/dichloromethane as eluent to give 4-chloro-2-trifluoromethylbenzonitrile (630 mg, 63% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.81-7.78 (m, 2H), 7.67 (dd, 1H, J = 8.4, 2.4 Hz).
Yield: 63%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 80;
Steps:
115
Add 4-chloro-l-iodo-2-trifluromethylbenzene (1500 mg, 4.89 ϖunol), zinc cyanide (345 mg, 2.94 mmol), and tetrakis(triρhenylphosphine)ρalladium(0) (564 mg, 0.488 mmol) to anhydrous N,N-dimethylformamide (40 mL). Heat to 80 0C overnight. Cool to room temperature, dilute with toluene, wash with 2 N ammonium hydroxide (3x), saturated aqueous sodium chloride, dry, filter, concentrate to give a residue. Chromatograph the residue on silica gel eluting with hexanes/dichloromethane, to give 4- chloro-2-trifluoromethylbenzonitrile (630 mg, 63%): 1H NMR (400 MHz, CDCl3) δ 7.81- 7.78 (m, 2H), 7.67 (dd, IH, J = 8.4, 2.4 Hz).
References:
ELI LILLY AND COMPANY WO2006/44454, 2006, A1 Location in patent:Page/Page column 63
108-20-3
589 suppliers
$25.00/25mL

23399-77-1
92 suppliers
$6.00/1g

320-41-2
148 suppliers
$10.00/1g
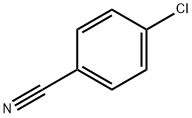
623-03-0
480 suppliers
$6.00/25g
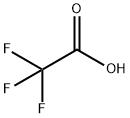
76-05-1
725 suppliers
$9.00/1g
1735-54-2
151 suppliers
$15.00/1g

320-41-2
148 suppliers
$10.00/1g