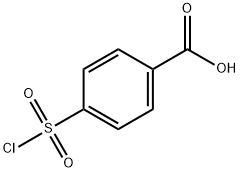
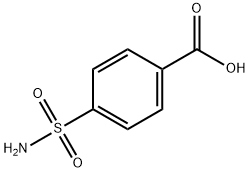
4-(CHLOROSULFONYL)BENZOIC ACID synthesis
- Product Name:4-(CHLOROSULFONYL)BENZOIC ACID
- CAS Number:10130-89-9
- Molecular formula:C7H5ClO4S
- Molecular Weight:220.63
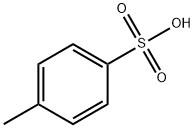
104-15-4

10130-89-9
The general procedure for the synthesis of 4-(chlorosulfonyl)benzoic acid from 4-methylphenylsulfonic acid was as follows: a solution of potassium permanganate (KMnO4, 1.6 g) in water (H2O, 13 mL) was added slowly and dropwise at 80 °C to a solution of 4-methylphenylsulfonic acid (1.7 g, 10 mmol) and potassium hydroxide (KOH, 0.68 g, 12 mmol) in water (H2O, a 15 mL) solution. Upon completion of the reaction, the mixture was poured into ethanol (EtOH, 20 mL), filtered and concentrated to give potassium 4-carboxybenzenesulfonate (1.2 g). This intermediate was used directly in the next reaction without further purification. At room temperature, chlorosulfonic acid (4 mL) was slowly added dropwise to a stirring solution of potassium 4-carboxybenzenesulfonate (1.2 g). The reaction mixture was continued to be stirred for 2 hours and then poured into crushed ice. The resulting solid product was collected by filtration and washed with water (H2O) to give 4-(chlorosulfonyl)benzoic acid (0.22 g) as a white solid in 20% yield. The melting point of the product was 230-232 °C (literature value: 228-232 °C); 1H-NMR (CDCl3, 400 MHz) data: δ 8.30 (d, J=8.0 Hz, 2H), 8.42 (d, J=8.0 Hz, 2H).

98-59-9
626 suppliers
$9.00/5g

10130-89-9
179 suppliers
$7.00/250mg
Yield:10130-89-9 55%
Reaction Conditions:
with chromium(VI) oxide;acetic anhydride;acetic acid at 20 - 40; for 2 h;
Steps:
30a
Step 30a: 4-(Chlorosulfonyl)benzoic acid (Compound 1402)To a solution of p-toluenesulfonyl chloride (1.9 g, 0.01 mol) in HAcZAc2O (20 ml/ 10 ml) was added chromium (VI) oxide (3.0 g, 0.03 mol) in portions at room temperature. The mixture was heated to 40°C for 2 h. and then the reaction mixture was poured into ice water, filtered, and dried to afford product 1402 (1.2 g, 55%): LCMS: 221 [M+l]+. 1H NMR (400MHz, DMSO-d6) δ 7.71 (d, J= 2.0 Hz, 2H), 7.92 (d, J= 6.0 Hz, 2H), 13.92 (s, IH).
References:
CURIS, INC. WO2009/36016, 2009, A1 Location in patent:Page/Page column 85

104-15-4
764 suppliers
$133.00/500g

10130-89-9
179 suppliers
$7.00/250mg
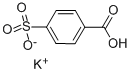
5399-63-3
88 suppliers
$17.67/5gm:

10130-89-9
179 suppliers
$7.00/250mg
150-13-0
946 suppliers
$5.00/25g

10130-89-9
179 suppliers
$7.00/250mg
138-41-0
308 suppliers
$11.00/25g

10130-89-9
179 suppliers
$7.00/250mg