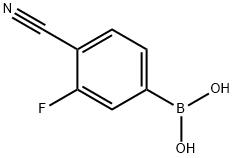
4-CYANO-3-FLUOROPHENYLBORONIC ACID synthesis
- Product Name:4-CYANO-3-FLUOROPHENYLBORONIC ACID
- CAS Number:843663-18-3
- Molecular formula:C7H5BFNO2
- Molecular Weight:164.93

5419-55-6
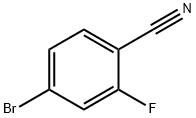
105942-08-3

843663-18-3
The general procedure for the synthesis of 4-cyano-3-fluorophenylboronic acid from triisopropyl borate and 4-bromo-2-fluorobenzonitrile was as follows: 4-bromo-2-fluorobenzonitrile (200 g, 990 mmol, 1.00 eq.) and triisopropyl borate (228 g, 1188 mmol, 1.2 eq.) were dissolved in a mixture of 700 mL tetrahydrofuran (THF) and 1400 mL toluene solvent. The reaction mixture was cooled to an internal temperature of -75 °C using a dry ice/acetone bath. n-Butyl lithium (n-BuLi, 396 mL of a 2.5 M hexane solution) was added slowly over a period of 2 hours. After the addition was completed, the reaction mixture appeared as a light red slurry. The reaction solution was continued to be stirred at -74 °C for 15 min, followed by a slow warming to -20 °C and the reaction was burst with 1500 mL of 2.5 M hydrochloric acid (HCl) solution. The reaction mixture was allowed to gradually warm up to room temperature (RT). The organic and aqueous layers were separated and the aqueous layer was extracted with ethyl acetate (EtOAc). The organic phases were combined, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to give a light brown solid. The solid was ground with hexane and transferred to a sintered glass funnel. It was washed again with hexane to give a light yellow filtrate. The light brown solid was mixed with cold dichloromethane (CH2Cl2) and stirred and filtered. The solid was washed with a small amount of dichloromethane to give an off-white solid and a brown filtrate. The solid was dried in a vacuum oven at 40°C to give a final 112 g (679 mmol, 69% yield) of 3-fluoro-4-cyanophenylboronic acid as an off-white solid.

5419-55-6
388 suppliers
$12.00/5g

105942-08-3
382 suppliers
$7.00/5g

843663-18-3
228 suppliers
$8.00/250mg
Yield:843663-18-3 69%
Reaction Conditions:
Stage #1: Triisopropyl borate;4-bromo-6-fluorobenzonitrilewith n-butyllithium in tetrahydrofuran;hexane;toluene at -75 - -74; for 2.25 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;hexane;toluene at -20 - 20;Product distribution / selectivity;
Steps:
A
Dissolve 2-Fluoro-4-bromobenzonitrile (200 g, 990 mmol, 1.00 eq.) and triisopropyl borate (228 g, 1188 mmol, 1.2 eq.) in 700 mL of THF and 1400 mL of toluene. Cool the mixture with a dry ice/acetone bath to an internal temperature of -75 0C. Slowly add n-BuLi (396 mL of a 2.5 M solution in hexanes) over a period of 2 hours. After addition is complete, a light-red, thin slurry occurs. Let the solution stir at -74 0C for 15 minutes, allow the solution to warm to -20 0C and then quench with 1500 mL of 2.5 M HCl. Let the solution warm to RT. Separate layers, extract the aqueous layer with EtOAc, dry the combined organic phases with Na2SQ,), filter and concentrate in vacuo to yield a light-brown solid.Triturate the solid with hexane and transfer to a scintered glass funnel. Rinse with hexane one more time to obtain a pale-yellow filtrate. Stir the light-brown solid with cold CH2Cl2 and filter. Rinse with a small volume of CH2Cl2 to yield an off-white solid and a brown filtrate. Dry the solid in a vacuum oven at 40 0C and dried to yield 112g (679 mmol, 69%) of 3-fluoro-4-cyanophenylboronic acid as an off-white solid.
References:
WO2007/87488,2007,A2 Location in patent:Page/Page column 17; 47-48

121-43-7
382 suppliers
$14.00/25mL

105942-08-3
382 suppliers
$7.00/5g

843663-18-3
228 suppliers
$8.00/250mg