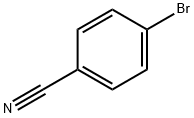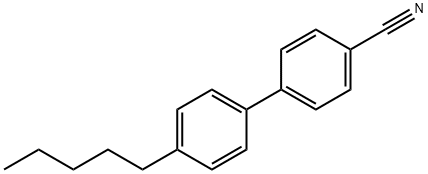
4-Cyano-4'-pentylbiphenyl synthesis
- Product Name:4-Cyano-4'-pentylbiphenyl
- CAS Number:40817-08-1
- Molecular formula:C18H19N
- Molecular Weight:249.35
63619-59-0

40817-08-1
General procedure for the synthesis of 4'-n-pentyl-4-cyanobiphenyl from p-bromopentyl biphenyl: To a dry flask containing magnesium shavings (0.29 g, 12 mmol) was added a solution of 1-bromo-4-methylbenzene (1.37 g, 8.0 mmol) in tetrahydrofuran (THF, 8 mL) at room temperature. After stirring the reaction mixture for 2 h, N,N-dimethylformamide (DMF, 1.3 mL, 12 mmol) was slowly added. The reaction mixture was cooled to 0°C and stirring was continued for 2 hours. Subsequently, ammonia solution (7 mL, 28-30%) and iodine (I2, 4.06 g, 16 mmol) were added to the reaction system. After stirring the reaction mixture for 2 h at room temperature, it was poured into saturated aqueous sodium sulfite (Na2SO3) solution and extracted with chloroform (CHCl3, 3 x 30 mL). The organic layers were combined, dried with anhydrous sodium sulfite (Na2SO4) and filtered. After the solvent was removed by distillation under reduced pressure, the residue was purified by silica gel short column chromatography (eluent: hexane/ethyl acetate = 9:1, v/v) to afford pure 4-methylbenzonitrile (0.77 g) in 82% yield.

63619-59-0
133 suppliers
$6.00/250mg

40817-08-1
262 suppliers
$34.00/1g
Yield:40817-08-1 80%
Reaction Conditions:
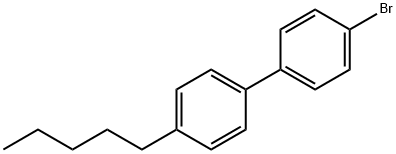
Stage #1: 4-(4-pentylphenyl)bromobenzenewith iodine;magnesium in tetrahydrofuran at 70;
Stage #2: with N,N-dimethyl-formamide in tetrahydrofuran at 0; for 2 h;
Stage #3: with ammonia;iodine in tetrahydrofuran;water at 20; for 2 h;
Steps:
3.2 Typical experimental procedure for conversion of aromaticbromides into aromatic nitriles with Mg, DMF, I2, and aqNH3
General procedure: To a flask containing Mg turnings (0.29 g, 12 mmol) was added1-bromo-4-methylbenzene (1.37 g, 8.0 mmol) in THF (8 mL) at room temperature. After being stirred for 2 h, DMF (1.3 mL,12 mmol) was added to the reaction mixture. The obtained mixturewas stirred for 2 h at 0 ° C. Then, aq NH3 (7 mL, 28-30%) and I2 (4.06 g, 16 mmol) were added to the reaction mixture. After beingstirred for 2 h at room temperature, the reaction mixture waspoured into satd aq Na2SO3 solution and was extracted with CHCl3 (3*30 mL). The organic layer was dried over Na2SO4 and filtered.After removal of the solvent, the residue was purified by shortcolumn chromatography on silica gel (eluent: hexane/ethylacetate=9:1, v/v) to provide pure 4-methyl-1-benzonitrile (0.77 g) in 82% yield.
References:
Ishii, Genki;Harigae, Ryo;Moriyama, Katsuhiko;Togo, Hideo [Tetrahedron,2013,vol. 69,# 5,p. 1462 - 1469]
630-18-2
197 suppliers
$10.00/1g

63619-59-0
133 suppliers
$6.00/250mg

40817-08-1
262 suppliers
$34.00/1g

124-38-9
134 suppliers
$214.00/14L

63619-59-0
133 suppliers
$6.00/250mg

40817-08-1
262 suppliers
$34.00/1g

63619-59-0
133 suppliers
$6.00/250mg
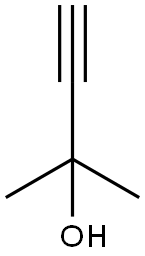
115-19-5
263 suppliers
$17.00/25mL

40817-08-1
262 suppliers
$34.00/1g