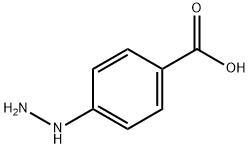
4-Hydrazinylbenzoic acid synthesis
- Product Name:4-Hydrazinylbenzoic acid
- CAS Number:619-67-0
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15
150-13-0
947 suppliers
$5.00/25g

619-67-0
436 suppliers
$6.00/5g
Yield:619-67-0 85.2%
Reaction Conditions:
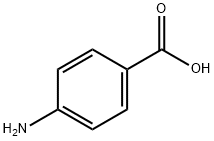
Stage #1:4-amino-benzoic acid with hydrogenchloride;sodium hydroxide;sodium nitrite in water at 0; for 0.833333 h;
Stage #2: with tin(II) chloride dihdyrate in water at 0 - 20;
Steps:
Synthesis of 4-hydrazinylbenzoic acid (1)
4-aminobenzoic acid (4.54 g, 33.1 mmol) was added to 1N NaOH (43 mL). The mixturewas added to 60 mL of 35% HCl at 0°C. NaNO2 (2.3 g. 33.3 mmol) in 20 mL H2O was addeddrop wise for 10 min. After stirring for 40 min at 0°C, SnCl2.2H2O (35.8 g, 158.7 mmol) in 35%HCl 72 mL was added over 30 min. The mixture was stirred at 0°C for 2 h, and then stirredovernight at room temperature. The collected precipitate on vacuum filtration and washed with100 mL diethyl ether, dried overnight in vacuum oven at 50°C to give the preferred product ascolorless solid, which was used without additional purification (3.87 g, 85.2 % yield). 1H NMR(600 MHz DMSO-d6): 7.009 (d, 2H, J= 9.0 Hz), 7.831 (d, 2H, J= 9.0 Hz), 8.944 (s, 1H), 10.629(s, 2H) (Fig. S1). 13C NMR (150 MHz DMSO-d6): 113.60, 123.27, 131.05, 149.96, 167.44. (Fig.S2). HRMS (m/z) calcd. for C7H9N2O2 [M+H]+: 153.0664, found 153.0654 (Fig. S3).
References:
Jayasudha, Palanisamy;Manivannan, Ramalingam;Park, Jong Ho;Son, Young-A [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2020,vol. 233] Location in patent:supporting information
586-76-5
504 suppliers
$10.00/1g

619-67-0
436 suppliers
$6.00/5g