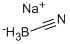
Sodium cyanoborohydride synthesis
- Product Name:Sodium cyanoborohydride
- CAS Number:25895-60-7
- Molecular formula:CH3BNNa
- Molecular Weight:62.84
Yield:25895-60-7 91%
Reaction Conditions:
with potassium hexamethylsilazane in tetrahydrofuran at 20; for 2 h;
Steps:
17
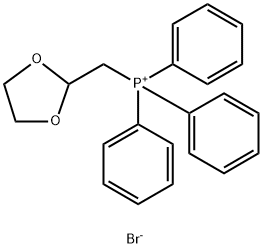
Example 17 (LS, 2S)-L- (3, 4-DICHLORO-PHENYL)-2- ( (Z/E)-2- [1, 3] dioxolan-2-yl-vinyl)- cyclopropanecarboxylic acid (4-FLUORO-BENZYL)-METHYL-AMIDE (1, 3-Dioxolan-2-ylmethyl) triphenylphosphonium bromide (3.39 g, 7.89 mmol) is suspended in dry THF (50 ml) under Argon and KHMDS (1.57 g, 7.89 mmol) is added portionwise at 0°C. The mixture is stirred at 0°C for 30 minutes, then allowed to warm to ambient temperature. (lS, 2R)-1- (3, 4-DICHLORO-PHENYL)-2-FORMYL-CYCLOPROPANECARBOXYLIC acid (4- fluoro-benzyl) -methyl-amide (1.0 g, 2.63 mmol) dissolved in dry THF (10 ml) is added dropwise and the mixture is stirred at ambient temperature for 2 hours. The reaction mixture is then poured onto ice cold water (30 ml) and the layers are separated. The aqueous phase is extracted with ethyl acetate (3 X 30 ml) and the combined organic fractions are washed with brine (sat. ), dried (MGS04), filtered and concentrated in vacuo. The crude product is purified by silica gel chromatography, eluting with a gradient of ethyl acetate: heptane (20: 80)- (50 : 50). The product is isolated as a Z/E isomer mixture. Yield 1.07 g (91%). LC/MS (ONLY) 450.1 (M+H+).
References:
WO2005/16884,2005,A1 Location in patent:Page/Page column 66-67
16940-66-2
0 suppliers
$21.21/100ml
592-04-1
0 suppliers
$245.00/10g
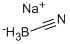
25895-60-7
526 suppliers
$9.00/1g
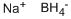
16940-66-2
0 suppliers
$21.21/100ml
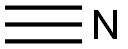
74-90-8
0 suppliers
inquiry
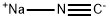
773837-37-9
0 suppliers
inquiry
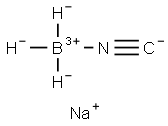
29012-60-0
0 suppliers
inquiry
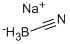
25895-60-7
526 suppliers
$9.00/1g
![Cyclopropanecarboxamide, 1-(3,4-dichlorophenyl)-N-[(4-fluorophenyl)methyl]-2-formyl-N-methyl-, (1S,2R)-](/CAS/20200515/GIF/846060-69-3.gif)