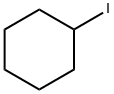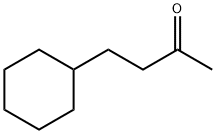
4-Cyclohexylbutane-2-one synthesis
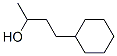
- Product Name:4-Cyclohexylbutane-2-one
- CAS Number:2316-85-0
- Molecular formula:C10H18O
- Molecular Weight:154.25
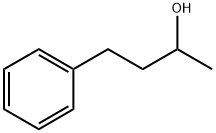
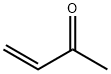
Yield:2316-85-0 91 %Chromat. ,2344-70-9 7 %Chromat.
Reaction Conditions:
with hydrogen in tetrahydrofuran at 30; under 15001.5 Torr; for 5 h;Catalytic behavior;Autoclave;Glovebox;
Steps:
3.3 General procedure for the catalytic hydrogenation reactions
General procedure: Autoclave Par 477 equipped with PID control temperature and reservoir for kinetic measurements and HEL 24 Cat reactor for substrate scope were used as reactors for the hydrogenation reactions. In a typical experiment, the autoclave was charged in the glove-box with the desired Rh NPs (1.25 or 0.625mol%; the catalyst concentration was calculated based on the total number of metallic Rh atoms in the surface of the NPs) and the substrate (0.124M) in THF. Molecular hydrogen was then introduced until the desired pressure was reached and the reaction was stirred for the desired reaction time at the selected temperature. At the end of the reaction, the autoclave was depressurised and the solution was filtered through silica for subsequent analysis by GC. The conversion and selectivities for each reaction product were determined by GC-FID on an Agilent Technologies 7890A spectrometer, with a HP-5 column (30m×0.25mm×0.25μm) using undecane as internal standard. TOF was defined as moles of products per mol Rh at the surface of the NPs per hour.
References:
Martinez-Espinar, Francisco;Blondeau, Pascal;Nolis, Pau;Chaudret, Bruno;Claver, Carmen;Castillón, Sergio;Godard, Cyril [Journal of Catalysis,2017,vol. 354,p. 113 - 127]
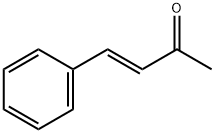
1896-62-4
233 suppliers
$11.00/100g
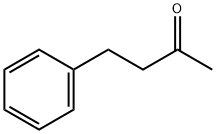
2550-26-7
370 suppliers
$9.00/100g

2316-85-0
8 suppliers
inquiry