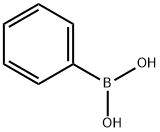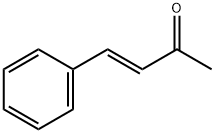
trans-4-Phenyl-3-buten-2-one synthesis
- Product Name:trans-4-Phenyl-3-buten-2-one
- CAS Number:1896-62-4
- Molecular formula:C10H10O
- Molecular Weight:146.19
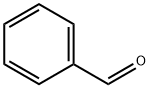
100-52-7
973 suppliers
$17.30/2g
67-63-0
1640 suppliers
$16.00/25ML

1896-62-4
232 suppliers
$11.00/100g
Yield:1896-62-4 80%
Reaction Conditions:
with caesium carbonate in water at 20;
Steps:
2.4. Procedure for synthesis of β-aryl enals and enones
General procedure: The β-aryl enals were synthesized by the following procedure.In a 50 mL round bottom (RB) flask, 2 mmol of benzaldehyde,9 mL ethanol (CH3CH2OH) and 3 mL water (H2O) was added in astoichiometric ratio of 3:1. After this, 2 equivalents of cesiumcarbonate (Cs2CO3) were added to the above mixture along with 25 mg of the synthesized CoCr2O4-HNT catalyst. The whole mixturewas then refluxed at 120 °C for 8 h. The reaction was monitoredthrough thin layer chromatography (TLC) and the productswere isolated through chromatography separation. Similarly, in a 50 mL RB flask b-aryl enones were synthesized bytaking the same amount of the substrate using 20 mg of CoCr2O4-HNT and in 1:3 ratio of H2O and ipr-OH (3 mL H2O + 9 mL ipr-OH).The reaction was stirred at room temperature for 4 h. After 4 h, thecatalyst was separated by normal filtration and the products wereseparated by chromatographic technique.
References:
Bania, Kusum K.;Baruah, Manash J.;Bhattacharyya, Pradip K.;Das, Biraj;Karunakar, Galla V.;Roy, Subhasish;Saikia, Lakshi;Saikia, Pinku;Sharma, Mukesh [Journal of Catalysis,2020,vol. 388,p. 104 - 121]
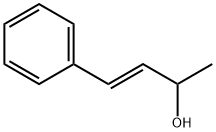
36004-04-3
4 suppliers
inquiry

1896-62-4
232 suppliers
$11.00/100g
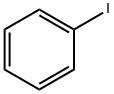
591-50-4
502 suppliers
$10.00/1g
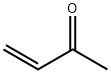
78-94-4
0 suppliers
$29.69/25ml

1896-62-4
232 suppliers
$11.00/100g
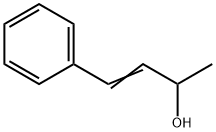
17488-65-2
22 suppliers
inquiry

1896-62-4
232 suppliers
$11.00/100g