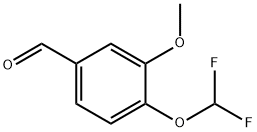
4-DIFLUOROMETHOXY-3-METHOXY-BENZALDEHYDE synthesis
- Product Name:4-DIFLUOROMETHOXY-3-METHOXY-BENZALDEHYDE
- CAS Number:162401-70-9
- Molecular formula:C9H8F2O3
- Molecular Weight:202.15
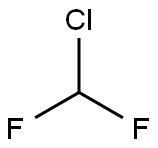
75-45-6
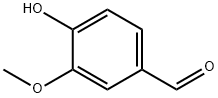
121-33-5

162401-70-9
1. DMF (450 mL), vanillin (30.0 g, 0.2 mol) and sodium hydroxide (13.0 g, 0.325 mol) were added to a reaction flask. 2. The reaction system was warmed to 90 °C with continuous stirring for 2 hours. 3. Monochlorodifluoromethane gas was introduced into the reaction system and the reaction progress was monitored by TLC until the vanillin was completely consumed. 4. Upon completion of the reaction, the heating was stopped and the reaction system was cooled to room temperature. 5. The reaction was quenched by addition of water (450 mL) and the reaction mixture was extracted with dichloromethane. 6. The organic phase was washed sequentially with saturated sodium carbonate solution and saturated brine, then dried over anhydrous sodium sulfate. 7. Concentrate the organic phase under pressure to obtain a light yellow oil 3-methoxy-4-(difluoromethoxy)benzaldehyde (31.9 g, 80% yield, 99.2% purity, HPLC chromatogram in Fig. 1), which can be used in subsequent steps without further purification.
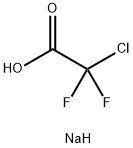
1895-39-2
294 suppliers
$6.00/5g

121-33-5
1171 suppliers
$9.00/1g

162401-70-9
134 suppliers
$15.00/1g
Yield: 91%
Reaction Conditions:
with caesium carbonate in DMF (N,N-dimethyl-formamide);water at 100; for 3.5 h;
Steps:
A solution of 4-hydroxy-3-methoxybenzaldehyde (2.0 g, 0.013 mol), sodium 2-chloro- 2, 2-difluoroacetate (4.8 g, 0.031 mol) and cesium carbonate (72 mg, 0.018 mol) in DMF (14 mL) and water (14 mL) was heated at 100°C for 3.5 hours. The mixture was acidified with concentrated hydrochloric acid and extracted with ethyl acetate (2x 25 mL). The organic layer was washed with water (2x25 mL), dried (MgSO4) and the solvent removed under reduced pressure. Purification by column chromatography with silica gel and ethyl acetate/n-hexane (1: 4) as eluent gave 4-(difluoromethoxy)-3-methoxybenzaldehyde (2.41 g, 91%) as an oil. 8 (250 MHz, CDCI3) : 3.76 (s, 3H); 6.49 (t, JF-H=74. 0 Hz, 1H) ; 7.11 (d, J=8.0 Hz, 1H) ; 7.27 (dd, Je=1. 7 Hz, J2=8.0 Hz, 1 H) ; 7.31 (d, J=1.7 Hz, 1 H) ; 9.74 (s, 1 H).
References:
ALMIRALL PRODESFARMA SA WO2005/58883, 2005, A1 Location in patent:Page/Page column 131
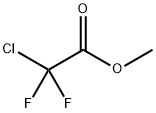
1514-87-0
261 suppliers
$9.00/1g

121-33-5
1171 suppliers
$9.00/1g

162401-70-9
134 suppliers
$15.00/1g
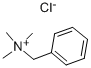
56-93-9
496 suppliers
$5.00/25g

121-33-5
1171 suppliers
$9.00/1g

162401-70-9
134 suppliers
$15.00/1g
74-87-3
221 suppliers
$18.50/48622
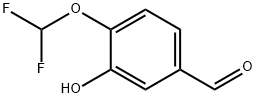
151103-08-1
278 suppliers
$8.00/250mg

162401-70-9
134 suppliers
$15.00/1g