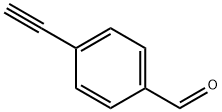
4-ETHYNYLBENZALDEHYDE synthesis
- Product Name:4-ETHYNYLBENZALDEHYDE
- CAS Number:63697-96-1
- Molecular formula:C9H6O
- Molecular Weight:130.14
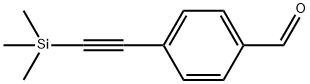
77123-57-0

63697-96-1
General procedure for the synthesis of 4-ethynylbenzaldehyde from 4-trimethylsilylethynylbenzaldehyde: Compound 4 (727 mg, 3.6 mmol) was dissolved in 15 mL of anhydrous THF, followed by the addition of 2 mL of aqueous KOH (298 mg, 4 mmol). The reaction mixture was stirred at room temperature for 2 hours. Upon completion of the reaction, the THF solvent was removed by rotary evaporator. The reaction mixture was extracted with ethyl acetate (EA) and washed three times with saturated NaCl solution. The combined organic phases were dried with anhydrous Na2SO4, filtered and concentrated. Finally, purification by column chromatography (eluent was petroleum ether and dichloromethane, 1:1, v/v) gave 450 mg of compound 5 as a light yellow solid in 97% yield.
1122-91-4
703 suppliers
$9.00/10g
1066-54-2
472 suppliers
$9.00/10g

63697-96-1
187 suppliers
$10.00/250mg
Yield:63697-96-1 85.1%
Reaction Conditions:
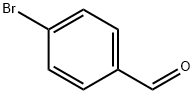
Stage #1:4-bromo-benzaldehyde with triethylamine in tetrahydrofuran for 0.75 h;Inert atmosphere;
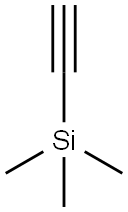
Stage #2:trimethylsilylacetylene with copper(l) iodide;triphenylphosphine hydrochloride in tetrahydrofuran for 10 h;Inert atmosphere;
Stage #3: with potassium carbonate in methanol at 20; for 5 h;
Steps:
1.3.a; 1.3.b
15.0 mmol (2.78 g) of p-bromobenzaldehyde was dissolved in 95 ml of a tetrahydrofuran and triethylamine mixed solution (1: 1 by volume) and placed in a 250 ml single-necked flask,The cells were ventilated for 45 minutes and then 18.0 mmol (1.76 g)Of trimethylsilylacetylene. Adding 97.2 mg of triphenylphosphonium dichloride,46.5mg cuprous iodide. Under argon, the reaction was carried out for 10 hours.After the reaction was stopped, the solvent was removed by distillation under reduced pressure and purified by silica gel column chromatography (developing solvent: dichloromethane: petroleum ether 1: 4), steamed and dried in vacuo to give 2.19 g of a white solid in 72.3% yield.B: A solution of 8.0 mmol (1.62 g) of a product was dissolved in 30 mlMethanol and 60 ml of tetrahydrofuran, placed in a 250 ml single-necked round bottom flask,Then add anhydrous potassium carbonate,And the mixture was stirred at room temperature for 5 hours. The solvent was distilled off under reduced pressure,Silica gel column (developing solvent for petroleum ether and dichloromethane 1: 1 mixed solvent) crude purification;Dried in vacuo to give 0.88 g of a white solid in 85.1%
References:
Xijing College;Zhao Yuzhen;Miao Zongcheng;Li Kexuan;Lin Dong;Ren Huaping;Zhu Min;Liu Kun CN106588789, 2017, A Location in patent:Paragraph 0047-0049

77123-57-0
113 suppliers
$15.00/1g

63697-96-1
187 suppliers
$10.00/250mg
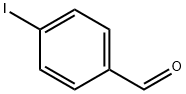
15164-44-0
294 suppliers
$6.00/1g

63697-96-1
187 suppliers
$10.00/250mg

1122-91-4
703 suppliers
$9.00/10g

63697-96-1
187 suppliers
$10.00/250mg
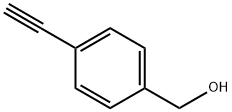
10602-04-7
126 suppliers
$24.00/100mg

63697-96-1
187 suppliers
$10.00/250mg