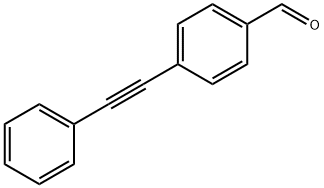
4-(2-PHENYLETH-1-YNYL)BENZALDEHYDE synthesis
- Product Name:4-(2-PHENYLETH-1-YNYL)BENZALDEHYDE
- CAS Number:57341-98-7
- Molecular formula:C15H10O
- Molecular Weight:206.24
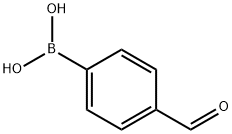
87199-17-5
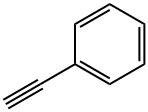
536-74-3

57341-98-7
4-Formylphenylboronic acid (0.5 mmol), phenylacetylene (0.6 mmol), dichloromethane (2 mL), Catalyst 3 (2 mol%), silver oxide (Ag2O, 1.0 mmol), and potassium acetate (KOAc, 1.0 mmol) were sequentially added to the Schlenk tube. The reaction mixture was stirred at 35 °C for 24 h under argon protection. Upon completion of the reaction, excess dichloromethane was removed by rotary evaporation and the residue was purified directly by silica gel column chromatography (eluent was petroleum ether or mixed petroleum ether/ethyl acetate solvent) to afford the target product, 4-(phenylalkynyl)benzaldehyde (32d, Table 1, entry 11) (99 mg, 96% yield). The product was a white solid, melting point: 98-100 °C; 1H NMR (400 MHz, CDCl3) δ 9.99 (s, 1H), 7.84 (d, J = 7.8 Hz, 2H), 7.66 (d, J = 8.0 Hz, 2H), 7.56 (dd, J = 3.4, 2.9 Hz, 2H), 7.37 (s, 3H); 13C NMR ( 100 MHz, CDCl3) δ 191.6, 135.3, 132.0, 131.7, 129.6, 128.9, 128.43, 128.41, 122.4, 93.5, 88.5.

536-74-3
316 suppliers
$10.00/1g
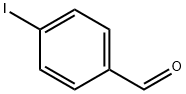
15164-44-0
294 suppliers
$6.00/1g

57341-98-7
103 suppliers
$30.00/100mg
Yield: 99%
Reaction Conditions:
with copper(l) iodide;C31H26N4PPdS(1+)*Cl(1-);sodium hydroxide in ethanol;toluene at 110; for 14 h;Catalytic behavior;Sonogashira Cross-Coupling;
Steps:
2.5.3. General procedure for Sonogashira coupling reactions
General procedure: To slurry of aryl halide (1 mmol), cuprous iodide (10 mol%) andpalladium catalyst (a known mol%) in an appropriate solvent(4 mL), phenylacetylene (1.2 mmol) and NaOH (1.7 mmol) wasadded and heated at required temp. After completion of the reaction(monitored by TLC), the flask was removed from the oil bathand water (20 mL) added, followed by extraction with ether(4 10 mL). The combined organic layers were washed with water(3 10 mL), dried over anhydrous Na2SO4, and filtered. Solventwas removed under vacuum. The residue was dissolved in hexaneand analyzed by GC-MS using Elite-5 columns, which are fused silicacapillary columns coated with 5% diphenyl and 95% dimethylpolysiloxane.
References:
Paul, Piyali;Butcher, Ray J.;Bhattacharya, Samaresh [Inorganica Chimica Acta,2015,vol. 425,p. 67 - 75]

87199-17-5
433 suppliers
$8.00/5g

536-74-3
316 suppliers
$10.00/1g

57341-98-7
103 suppliers
$30.00/100mg
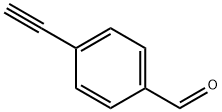
63697-96-1
187 suppliers
$10.00/250mg

98-80-6
743 suppliers
$5.00/5g

57341-98-7
103 suppliers
$30.00/100mg
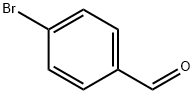
1122-91-4
702 suppliers
$9.00/10g

536-74-3
316 suppliers
$10.00/1g

57341-98-7
103 suppliers
$30.00/100mg
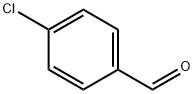
104-88-1
646 suppliers
$5.00/25g

536-74-3
316 suppliers
$10.00/1g

57341-98-7
103 suppliers
$30.00/100mg