4-fluoro-1-nitro-2-phenylbenzene synthesis
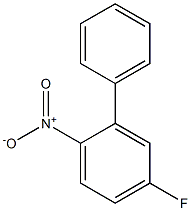
- Product Name:4-fluoro-1-nitro-2-phenylbenzene
- CAS Number:1478-01-9
- Molecular formula:C12H8FNO2
- Molecular Weight:217.1958
Yield:1478-01-9 99%
Reaction Conditions:
with potassium carbonate in ethanol;water;toluene; for 6 h;Reflux;
Steps:
10 Preparation of compound 10-1
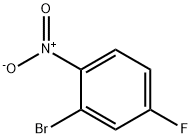
Preparation of compound 10-1 After introducing 2-bromo-4-fluoro-1-nitrobenzene (50 g, 227.3 mmol), phenyl boronic acid (30.5 g, 250 mmol), Pd(PPh3)4(13.1 g, 11.37 mmol), K2CO3(62.8 g, 454.6 mmol), toluene 600 mL, EtOH 200 mL, and purified water 200 mL in a reaction container, the mixture was stirred under reflux for 6 hours. After cooling the mixture to room temperature, an organic layer was extracted with ethylacetate (EA) and distilled water. The obtained organic layer was distilled under reduced pressure, and the residue was separated with column chromatography to obtain compound 10-1 (49 g, 99%).
References:
WO2015/178731,2015,A1 Location in patent:Paragraph 258; 259; 260; 261

108-86-1
496 suppliers
$10.00/5g

1478-01-9
4 suppliers
inquiry
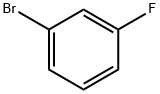
1073-06-9
427 suppliers
$6.00/10g

1478-01-9
4 suppliers
inquiry