4-FLUORO-2-NITRO-BIPHENYL synthesis
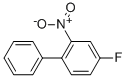
- Product Name:4-FLUORO-2-NITRO-BIPHENYL
- CAS Number:390-06-7
- Molecular formula:C12H8FNO2
- Molecular Weight:217.1958
Yield:390-06-7 82%
Reaction Conditions:
with tetrabutylammomium bromide;palladium diacetate;sodium carbonate in water at 165; for 0.125 h;Microwave irradiation;
Steps:
3 Preparation 3
4-Fluoro-2-nitro-1,1'-biphenyl
Preparation 3
4-Fluoro-2-nitro-1,1'-biphenyl
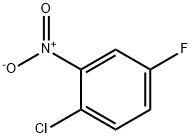
A microwave reaction vessel was charged with 2-chloro-5-fiuoronitrobenzene (0.175 g, 1 mmol), phenylboronic acid (0.134 g, 1.1 mmol), sodium carbonate (0.317 g, 3 mmol), palladium diacetate (0.009 g, 0.04 mmol), tetrabutylammonium bromide (0.322 g, 1 mmol) in water (2 mL).
The mixture was heated to 165° C. in a microwave reactor for 7.5 minutes.
The reaction was cooled and poured into ether and 0.1N aqueous sodium hydroxide.
The ether layer was washed with saturated aqueous sodium chloride, dried (anhydrous sodium sulfate), filtered, and concentrated.
The crude product was purified by silica gel column chromatography (5-30% methylene chloride in hexanes) to give the desired product as a pale yellow oil (0.179 g, 82%).
1H NMR (CDCl3, 300 MHz) δ 7.62-7.58 (dd, 1H, J=2.7, 8.1 Hz), 7.46-7.40 (m, 4H), 7.38-7.34 (m, 1H), 7.31-7.26 (m, 2H). HPLC analysis (C18, 5-95% acetonitrile in H2O+0.1% trifluoroacetic acid over 20 min: retention time, % area at 254 nm): 9.95 min, 96.3%.
References:
US2013/303524,2013,A1 Location in patent:Paragraph 0242-0243

108-86-1
523 suppliers
$10.00/5g

390-06-7
10 suppliers
inquiry