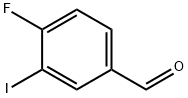
4-FLUORO-3-IODOBENZALDEHYDE synthesis
- Product Name:4-FLUORO-3-IODOBENZALDEHYDE
- CAS Number:227609-88-3
- Molecular formula:C7H4FIO
- Molecular Weight:250.01
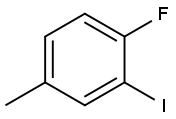
452-82-4
120 suppliers
$15.00/1g

227609-88-3
86 suppliers
$38.00/250mg
Yield: 41.5%
Reaction Conditions:
Stage #1:1-fluoro-2-iodo-4-methylbenzene with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane for 3 h;Heating / reflux;
Stage #2: with sodium hydrogencarbonate in dimethyl sulfoxide at 120; for 1.5 h;
Steps:
8.8A
A mixture of 4-fluoro-3-iodotoluene (5.0 g, 21.2 mmol) and NBS (4.2 g, 23.3 mmol) in 50 mL of CCl 4 was refluxed under N 2 with benzoyl peroxide (250 mg, 1.03 mmol) was heated for 3 hours. The reaction mixture was cooled to room temperature and filtered through celite, washed with benzene. The filtrate was evaporated and pumped to give the benzylbromide as a crude light brown oil. [0192] A mixture of benzylbromide in 50 mL of DMSO was heated with NaHCO 3 solid (3.55 g, 42.2 mmol) at 120° C. for 90 min. The reaction mixture was then cooled to room temperature, quenched with water, extracted with Et 2O, and washed with water, brine. The organic layer was dried with Na 2SO 4, concentrated in vacuuo. MPLC purification provided the titled compound as a colorless oil which solidified over time (2.2 g, 41.5% over two steps).
References:
Xin, Zhili;Liu, Gang;Pei, Zhonghua;Szczepankiewicz, Bruce G.;Serby, Michael D.;Zhao, Hongyu US2004/214870, 2004, A1 Location in patent:Page 22
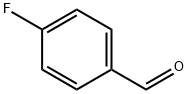
459-57-4
602 suppliers
$6.00/25g

227609-88-3
86 suppliers
$38.00/250mg
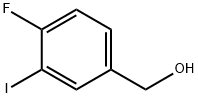
227609-87-2
32 suppliers
$93.00/250mg

227609-88-3
86 suppliers
$38.00/250mg
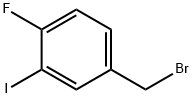
260050-97-3
14 suppliers
$128.00/250 mg

227609-88-3
86 suppliers
$38.00/250mg
2365-85-7
199 suppliers
$9.00/1g

227609-88-3
86 suppliers
$38.00/250mg