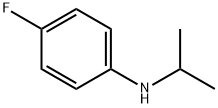
4-FLUORO-N-ISOPROPYLANILINE synthesis
- Product Name:4-FLUORO-N-ISOPROPYLANILINE
- CAS Number:70441-63-3
- Molecular formula:C9H12FN
- Molecular Weight:153.2
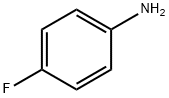
371-40-4

70441-63-3
General procedure for the synthesis of 4-fluoro-N-isopropylaniline from 4-fluoroaniline: 22.2 g (0.2 mol) of 4-fluoroaniline and 17.6 mL (0.24 mol) of acetone were added to a 500 mL three-necked, round-bottomed flask, dissolved in 120 mL (2 mol) of glacial acetic acid, mechanically stirred, and cooled in an ice bath to about 10°C. The mixture was then purified to about 10°C with mechanical stirring. 9.4 g (0.25 mol) of sodium borohydride was added in batches while keeping the temperature below 20°C. After addition, the reaction mixture was stirred at 20°C for 30 minutes to 1 hour. Subsequently, the mixture was poured into 500 mL of ice water. The aqueous solution was made alkaline by dropwise addition of 120 mL of 50% aqueous sodium hydroxide (keeping the temperature below 25°C). The product was extracted with hexane (2 x 100 mL) and the organic phases were combined, washed with saturated sodium chloride solution and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to give a clarified liquid. The product was purified by distillation and the fraction with a boiling point of 40 °C/0.5 mmHg was collected to give 21.8 g of clarified colorless liquid product in 71% yield.
350-46-9
522 suppliers
$10.60/1gm:

70441-63-3
139 suppliers
$34.00/250mg
Yield:70441-63-3 97%
Reaction Conditions:
Pt on charcoal in water;acetone;toluene
Steps:
1.a Preparation of 4-fluoro-N-isopropylaniline
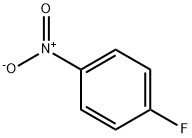
Example 1a Preparation of 4-fluoro-N-isopropylaniline 520 parts of toluene, 423 parts (3 mol) of 4-fluoronitrobenzene, 192 parts (3.3 mol) of acetone and 8 parts of catalyst (5% Pt on charcoal, water-moist, water content 50%) are placed in a hydrogenation autoclave. After the gas space has been flushed three times with each of nitrogen and hydrogen, the mixture is heated to from 80°=0 to 85° C. with stirring and reduced at a hydrogen pressure of from 0.2 to 1.0 MPa. When after about 2 hours the uptake of hydrogen falls sharply, the pressure is raised to 2.0 MPa and stirring is continued at 85° C for 30 minutes until there is no longer any drop in pressure. The batch is cooled to 60° C., the autoclave is let down and flushed with nitrogen, and the catalyst is filtered off on a pressure filter at from 50° to 60° C. The aqueous phase is separated off from the filtrate and toluene is distilled off until the water content of the solution is <0.02%. The solution is processed further in accordance with Example 2. Alternatively, the solution can be worked up by distillation and the product isolated. Yield 97-98%; purity by GC: ≥99.2%; boiling point: 91° to 92° C./10 torr.
References:
Hoechst Aktiengesellschaft US5616799, 1997, A

350-46-9
522 suppliers
$10.60/1gm:
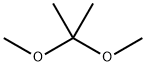
77-76-9
398 suppliers
$9.00/5ml

70441-63-3
139 suppliers
$34.00/250mg

350-46-9
522 suppliers
$10.60/1gm:

67-64-1
0 suppliers
$17.30/10ml

70441-63-3
139 suppliers
$34.00/250mg

371-40-4
406 suppliers
$5.00/5 g

70441-63-3
139 suppliers
$34.00/250mg
108-18-9
385 suppliers
$10.00/1g

371-40-4
406 suppliers
$5.00/5 g

70441-63-3
139 suppliers
$34.00/250mg