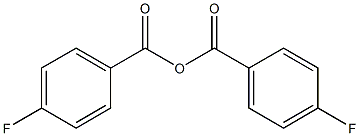
4-FLUOROBENZOIC ANHYDRIDE synthesis
- Product Name:4-FLUOROBENZOIC ANHYDRIDE
- CAS Number:25569-77-1
- Molecular formula:C14H8F2O3
- Molecular Weight:262.21
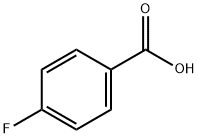
456-22-4

25569-77-1
GENERAL METHOD: p-Fluorobenzoic acid (1 mmol) was dissolved in anhydrous CH3CN (5 mL) with K2CO3 (1.5 mmol, 0.20 g), followed by the addition of TsCl (0.5 mmol, 0.10 g). The reaction mixture was stirred at room temperature for appropriate time (20-180 min, see Table 2) until TLC detection showed complete consumption of TsCl. After completion of the reaction, CH2Cl2 or Et2O (2 × 10 mL) was added to the mixture, stirred thoroughly and filtered. The organic layer was dried with CaCl2 and the solvent was subsequently evaporated to give high purity 4-fluorobenzoic anhydride.

456-22-4
496 suppliers
$10.00/100 g

25569-77-1
61 suppliers
$13.00/250mg
Yield:25569-77-1 95%
Reaction Conditions:
with potassium carbonate;p-toluenesulfonyl chloride in acetonitrile at 20; for 2.5 h;
Steps:
3.2. General Procedure for the Preparation of CarboxylicAcid Anhydride Using TsCl in Conventional System
General procedure: TsCl (0.5 mmol, 0.10 g) was added to the mixture of carboxylic acid (1 mmol) and K2CO3 (1.5 mmol, 0.20 g) in dry CH3CN (5 mL), the reaction mixture was stirred at roomtemperature for the appropriate time (20-180 min, Table 2) until the TsCl was no longer detectable by TLC. After the completion of the reaction, CH2Cl2 or Et2O (2×10 mL) was added to the reaction mixture, stirred, filtered, and the organic layer was dried over CaCl2. The evaporation of the solvent afforded a highly pure product.
References:
Eskandari, Parvin;Kazemi, Foad [Letters in Organic Chemistry,2017,vol. 14,# 6,p. 431 - 439]
403-43-0
478 suppliers
$9.00/25g

25569-77-1
61 suppliers
$13.00/250mg
459-57-4
602 suppliers
$6.00/25g

25569-77-1
61 suppliers
$13.00/250mg
352-34-1
381 suppliers
$7.00/5g
201230-82-2
1 suppliers
inquiry

25569-77-1
61 suppliers
$13.00/250mg

456-22-4
496 suppliers
$10.00/100 g

403-43-0
478 suppliers
$9.00/25g

25569-77-1
61 suppliers
$13.00/250mg