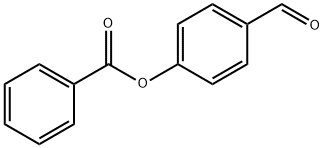
4-FORMYLPHENYL BENZOATE synthesis
- Product Name:4-FORMYLPHENYL BENZOATE
- CAS Number:5339-06-0
- Molecular formula:C14H10O3
- Molecular Weight:226.23
Yield:5339-06-0 98%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
Representative procedure for the preparation of intermediates 2a - i.
General procedure: 4-formylphenyl propionate (2b) is used as an example. To a solution of 4-hydroxybenzaldehyde (1) (1.0 g, 8.19 mmol) in THF (25 mL) at 0 oC was added Et3N (1.71 mL, 12.2 mmol) followed by propionyl chloride (1.06 mL, 12.2 mmol). The reaction mixture was warmed to room temperature and stirred for 3 h. At this point, the reaction mixture was diluted with EtOAc and quenched with aqueous NH4Cl. The aqueous layer was extracted with EtOAc (2) and the combined organic layers were subsequently washed with H2O and brine, and concentrated in vacuo. The resulting residue was purified by column chromatography (hexanes/EtOAc, 3:1) to provide compound 2b as a colorless oil.
References:
Zakhari, Joseph S.;Kinoyama, Isao;Hixon, Mark S.;Di Mola, Antonia;Globisch, Daniel;Janda, Kim D. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 21,p. 6203 - 6209] Location in patent:supporting information; experimental part
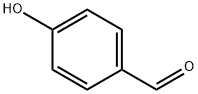
123-08-0
964 suppliers
$5.00/10g
65-85-0
1441 suppliers
$10.00/25g

5339-06-0
20 suppliers
$60.00/50mg

798544-46-4
0 suppliers
inquiry

5339-06-0
20 suppliers
$60.00/50mg
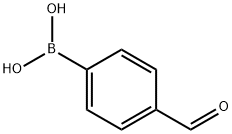
87199-17-5
435 suppliers
$8.00/5g
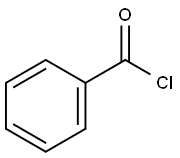
98-88-4
595 suppliers
$10.00/5g

5339-06-0
20 suppliers
$60.00/50mg